Page 52 - Read Online
P. 52
Page 8 of 35 Villeda-Hernandez et al. Soft Sci 2024;4:14 https://dx.doi.org/10.20517/ss.2023.52
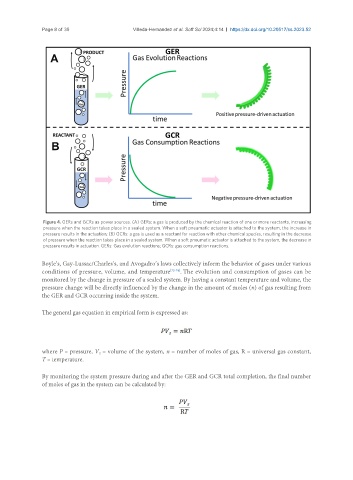
Figure 4. GERs and GCRs as power sources. (A) GERs: a gas is produced by the chemical reaction of one or more reactants, increasing
pressure when the reaction takes place in a sealed system. When a soft pneumatic actuator is attached to the system, the increase in
pressure results in the actuation; (B) GCRs: a gas is used as a reactant for reaction with other chemical species, resulting in the decrease
of pressure when the reaction takes place in a sealed system. When a soft pneumatic actuator is attached to the system, the decrease in
pressure results in actuation. GERs: Gas evolution reactions; GCRs: gas consumption reactions.
Boyle’s, Gay-Lussac/Charles’s, and Avogadro’s laws collectively inform the behavior of gases under various
conditions of pressure, volume, and temperature [72-74] . The evolution and consumption of gases can be
monitored by the change in pressure of a sealed system. By having a constant temperature and volume, the
pressure change will be directly influenced by the change in the amount of moles (n) of gas resulting from
the GER and GCR occurring inside the system.
The general gas equation in empirical form is expressed as:
where P = pressure, V = volume of the system, n = number of moles of gas, R = universal gas constant,
S
T = temperature.
By monitoring the system pressure during and after the GER and GCR total completion, the final number
of moles of gas in the system can be calculated by: