Page 49 - Read Online
P. 49
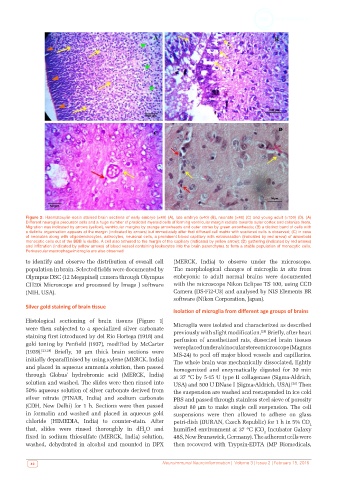
Figure 2: Haematoxylin-eosin stained brain sections of early embryo (×40) (A), late embryo (×40) (B), neonate (×40) (C) and young adult (×100) (D). (A)
Different neuroglia precursor cells and a huge number of predicted myeloid cells of forming ventricular margin radiate towards outer cortex and colonize there.
Migration was indicated by arrows (yellow), ventricular margins by orange arrowheads and outer cortex by green arrowheads; (B) a distinct band of cells with
a definite organisation appears at the margin (indicated by arrows) but immediately after that diffused cell matrix with scattered cells is observed; (C) in case
of neonates along with oligodendrocytes, astrocytes, neuronal cells, a prominent blood capillary with extravasation (indicated by red arrow) of amoeboid
monocytic cells out of the BBB is visible. A cell also tethered to the margin of the capillary (indicated by yellow arrow); (D) gathering (indicated by red arrows)
and infiltration (indicated by yellow arrows) of blood vessel containing leukocytes into the brain parenchyma to form a stable population of monocytic cells.
Perivascular macrophage/microglia are also observed
to identify and observe the distribution of overall cell (MERCK, India) to observe under the microscope.
population in brain. Selected fields were documented by The morphological changes of microglia in situ from
Olympus DSC (12 Megapixel) camera through Olympus embryonic to adult normal brains were documented
CH20i Microscope and processed by Image J software with the microscope Nikon Eclipse TS 100, using CCD
(NIH, USA). Camera (DS-Fi2-U3) and analysed by NIS Elements BR
software (Nikon Corporation, Japan).
Silver gold staining of brain tissue
Isolation of microglia from different age groups of brains
Histological sectioning of brain tissues [Figure 1]
were then subjected to a specialized silver carbonate Microglia were isolated and characterized as described
previously with slight modification. Briefly, after heart
[25]
staining first introduced by del Rio Hortega (1918) and perfusion of anesthetized rats, dissected brain tissues
gold toning by Penfield (1937), modified by McCarter were placed under a binocular stereomicroscope (Magnus
(1939). [23,24] Briefly, 10 µm thick brain sections were MS-24) to peel off major blood vessels and capillaries.
initially deparaffinised by using xylene (MERCK, India) The whole brain was mechanically dissociated, lightly
and placed in aqueous ammonia solution, then passed homogenized and enzymatically digested for 30 min
through Globus’ hydrobromic acid (MERCK, India) at 37 ºC by 5-15 U type II collagenase (Sigma-Aldrich,
solution and washed. The slides were then rinsed into USA) and 500 U DNase I (Sigma-Aldrich, USA). Then
[26]
50% aqueous solution of silver carbonate derived from the suspension are washed and resuspended in ice cold
silver nitrate (FINAR, India) and sodium carbonate PBS and passed through stainless steel sieve of porosity
(CDH, New Delhi) for 1 h. Sections were then passed about 80 µm to make single cell suspension. The cell
in formalin and washed and placed in aqueous gold suspensions were then allowed to adhere on glass
chloride (HIMEDIA, India) to counter-stain. After petri-dish (DURAN, Czech Republic) for 1 h in 5% CO 2
that, slides were rinsed thoroughly in dH O and humified environment at 37 ºC (CO Incubator Galaxy
2 2
fixed in sodium thiosulfate (MERCK, India) solution, 48S, New Brunswick, Germany). The adherent cells were
washed, dehydrated in alcohol and mounted in DPX then recovered with Trypsin-EDTA (MP Biomedicals,
40 Neuroimmunol Neuroinflammation | Volume 3 | Issue 2 | February 15, 2016