Page 51 - Read Online
P. 51
Page 12 of 30 Yoon et al. Energy Mater 2024;4:400063 https://dx.doi.org/10.20517/energymater.2023.146
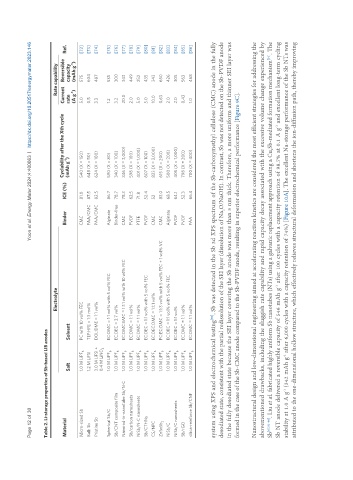
Table 2. Li-storage properties of Sb-based LIB anodes
Electrolyte Rate capability
Cyclability after the Xth cycle Current Reversible
Material Binder ICE (%) -1 Ref.
Salt Solvent (mAh g ) rate capacity
-1
-1
(A g ) (mAh g )
Micro-sized Sb 1.0 M LiPF 6 PC with 10 vol% FEC CMC 81.0 540 (X = 150) 5.0 575 [72]
Bulk Sb 1.2 M LiFSI TEP:HFE = 1:3 mol% PAA/CMC 87.5 648 (X = 50) 0.5 604 [73]
Pristine Sb 3.0 M LiFSI + DOL:DME = 1:1 vol% PAA/CMC 82.5 624 (X = 100) 3.3 487 [74]
0.4 M LiNO
3
Spherical Sb/C 1.0 M LiPF 6 EC:DMC = 1:1 vol% with 5 vol% FEC Alginate 86.7 590 (X = 80) 1.2 535 [75]
Sb/CNT composite film 1.0 M LiPF EC:DEC = 3:7 vol% Binder-free 78.7 340 (X = 100) 3.2 300 [76]
6
Nanorod-in-nanotube Sb/N-C 1.0 M LiPF 6 EC:DMC:EMC = 1:1:1 vol% with 10 wt% FEC CMC 78.3 346 (X = 3,000) 20.0 343 [77]
Sb/carbon nanosheets 1.0 M LiPF EC:DMC = 1:1 vol% PVDF 62.5 598 (X = 100) 2.0 449 [78]
6
NiSb/N-C nanosheets 1.0 M LiPF 6 EC:DMC = 1:1 vol% PTFE 71.8 401 (X = 1,000) 5.0 252 [79]
Sb/CTHNs 1.0 M LiPF EC:DEC = 1:1 vol% with 5 vol% FEC PVDF 52.4 607 (X = 100) 5.0 435 [80]
6
CS/NPC 1.0 M LiPF 6 EC:DEC:DMC = 1:1:1 vol% CMC 52 833 (X = 3,000) 10.0 343 [81]
ZnSnSb 2 1.0 M LiPF 6 PC:EC:DMC = 1:1:3 vol% with 5 vol% FEC + 1 vol% VC CMC 83.0 615 (X = 200) 0.63 650 [82]
NiSb/C 1.0 M LiPF EC:DEC = 1:1 vol% with 5 vol% FEC Alginate 68.5 500 (X = 200) 2.0 426 [83]
6
NiSb/C nanosheets 1.0 M LiPF 6 EC:DEC = 1:1 vol% PVDF 64.1 405 (X = 1,000) 2.0 305 [84]
Sb/rGO 1.0 M LiPF EC:DMC = 1:1 vol% PVDF 52.3 798 (X = 200) 0.43 563 [85]
6
silica-reinforce Sb/CNF 1.0 M LiPF 6 EC:DMC = 1:1 vol% PAA 66.4 700 (X = 400) 1.0 468 [86]
[90]
system using XPS and electrochemical testing . Sb was detected in the Sb 3d XPS spectrum of the Sb-carboxymethyl cellulose (CMC) anode in the fully
desodiated state, consistent with the partial redissolution of the SEI layer (dissolution of Na O/NaOH). In contrast, Sb was not detected on the Sb-PVDF anode
2
in the fully desodiated state because the SEI layer covering the Sb anode was more than 5 nm thick. Therefore, a more uniform and thinner SEI layer was
formed in the case of the Sb-CMC anode compared to the Sb-PVDF anode, resulting in superior electrochemical performance [Figure 9C].
Nanostructural design and low-dimensional engineering aimed at accelerating reaction kinetics are considered the most efficient strategies for addressing the
abovementioned drawbacks, including the sluggish rate capability and rapid capacity decay associated with the excessive volume change experienced by
Sb [67,91-96] . Liu et al. fabricated highly uniform Sb nanotubes (NTs) using a galvanic replacement approach using a Cu Sb-mediated formation mechanism . The
[91]
2
Sb NT anode delivered a reversible capacity of 546 mAh g after 100 cycles with a capacity retention of 98.7% at 0.1 A g and excellent long-term cycling
-1
-1
-1
-1
stability at 1.0 A g (342 mAh g after 6,000 cycles with a capacity retention of 74%) [Figure 10A]. The excellent Na-storage performance of the Sb NTs was
attributed to the one-dimensional hollow structure, which effectively relieves structural deformation and shortens the ion-diffusion path, thereby improving