Page 56 - Read Online
P. 56
Yoon et al. Energy Mater 2024;4:400063 https://dx.doi.org/10.20517/energymater.2023.146 Page 17 of 30
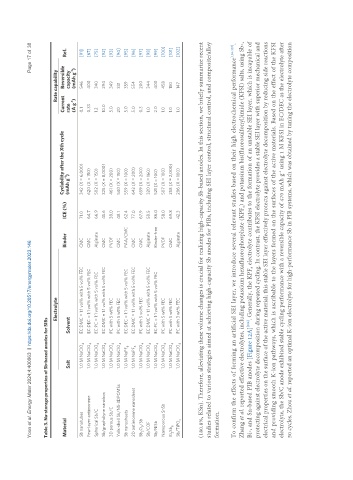
Table 3. Na-storage properties of Sb-based anodes for SIBs
Electrolyte Rate capability
Cyclability after the Xth cycle Current Reversible
Material Binder ICE (%) -1 Ref.
Salt Solvent (mAh g ) rate capacity
-1
-1
(A g ) (mAh g )
Sb nanotubes 1.0 M NaClO 4 EC:DMC = 1:1 vol% with 5 vol% FEC CMC 71.0 342 (X = 6,000) 0.1 546 [91]
Few-layer antimonene 1.0 M NaClO EC:DEC = 1:1 vol% with 5 vol% FEC CMC 64.7 620 (X = 150) 0.33 400 [67]
4
Spherical Sb/C 1.0 M NaClO 4 EC:PC = 1:1 vol% with 5 vol% FEC Alginate 66.9 502 (X = 150) 1.2 340 [75]
Sb/graphdiyne nanobox 1.0 M NaClO EC:DMC = 1:1 vol% with 5 vol% FEC CMC 45.6 325 (X = 8,000) 10.0 294 [92]
4
3D porous Sb/C 1.0 M NaClO 4 PC with 5 wt% FEC PVDF 38.0 461 (X = 200) 5.0 349 [93]
Yolk-shell Sb/NS-3DPCMSs 1.0 M NaClO PC with 5 wt% FEC CMC 48.1 540 (X = 150) 20 331 [94]
4
Sb nanosheets 1.0 M NaPF 6 EC:DEC = 1:1 vol% with 5 vol% FEC PAA/CMC 62.4 559 (X = 100) 5.0 359 [95]
2D antimonene nanosheet 1.0 M NaPF EC:DMC = 1:1 vol% with 5 vol% FEC CMC 77.0 642 (X = 200) 5.0 554 [96]
6
Sb O /Sb 1.0 M NaClO 4 PC with 5 vol% FEC CMC 67.9 659 (X = 200) 0.3 200 [97]
2
3
Sb/COF 1.0 M NaClO EC:DMC = 1:1 vol% with 5 vol% FEC Alginate 58.5 320 (X = 160) 1.0 344 [98]
4
Sb/NiSb 1.0 M NaClO 4 EC:PC = 1:1 vol% with 5 vol% FEC Binder-free 86.0 521 (X = 100) 2.0 400 [99]
Nanoporous SnSb 1.0 M NaClO 4 PC with 5 wt% FEC PVDF 58.0 507 (X = 100) 1.0 458 [100]
Bi Sb 1.0 M NaClO PC with 5 wt% FEC CMC 69.8 258 (X = 2,000) 1.0 150 [101]
2 6 4
Sb/TiPO x 1.0 M NaClO 4 PC with 3 wt% FEC Alginate 42.3 286 (X = 100) 1.0 147 [102]
(180.8%, KSn). Therefore, alleviating these volume changes is crucial for realizing high-capacity Sb-based anodes. In this section, we briefly summarize recent
studies related to various strategies aimed at achieving high-capacity Sb anodes for PIBs, including SEI layer control, structural control, and composite/alloy
formation.
To confirm the effects of forming an artificial SEI layer, we introduce several relevant studies based on their high electrochemical performance [105-107] .
Zhang et al. reported effective electrolytes, including potassium hexafluorophosphate (KPF ) and potassium bis(fluorosulfonyl)imide (KFSI) salts, using Sb-,
6
Bi-, and Sn-based PIB anodes [Figure 12A] . Generally, the KPF electrolyte contributes to the formation of an unstable SEI layer, which is incapable of
[105]
6
protecting against electrolyte decomposition during repeated cycling. In contrast, the KFSI electrolyte provides a stable SEI layer with superior mechanical and
electrical properties on the surface of the active material; this stable SEI layer effectively protects against electrolyte decomposition by reducing side reactions
and providing smooth K-ion pathways, which is ascribable to the layers formed on the surfaces of the active materials. Based on the effect of the KFSI
electrolyte, the Sb/C anode exhibited stable cycling performance with a reversible capacity of 470 mAh g using 1 M KFSI in EC/DEC as the electrolyte after
-1
50 cycles. Zhou et al. reported an optimal K-ion electrolyte for high-performance Sb in PIB systems, which was obtained by tuning the electrolyte composition