Page 61 - Read Online
P. 61
Page 22 of 30 Yoon et al. Energy Mater 2024;4:400063 https://dx.doi.org/10.20517/energymater.2023.146
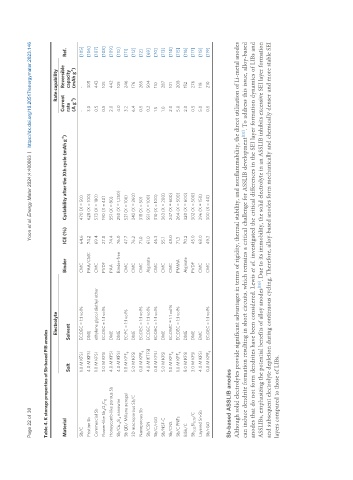
Table 4. K storage properties of Sb-based PIB anodes
Electrolyte Rate capability
-1
Material Binder ICE (%) Cyclability after the Xth cycle (mAh g ) Current Reversible Ref.
Salt Solvent rate capacity
-1
-1
(A g ) (mAh g )
Sb/C 1.0 M KFSI EC:DEC = 1:1 vol% CMC 64.6 470 (X = 50) - - [105]
Pristine Sb 4.0 M KFSI DME PAA/CMC 74.2 628 (X = 100) 3.0 305 [106]
Commercial Sb 1.0 M KFSI ethylene glycol diethyl ether CMC 69.4 573 (X = 180) 0.5 443 [107]
Flower-like Sb O C 3.0 M KFSI EC:DEC = 1:1 vol% PVDF 27.8 190 (X = 40) 0.5 105 [108]
4 5 l2
Honeycomb-like porous Sb 4.0 M KFSI DME PAA 74.4 551 (X = 80) 2.0 442 [109]
Sb/Cu Si nanowire 4.0 M KFSI DME Binder-free 76.0 250 (X = 1,250) 4.0 105 [110]
15 4
Sb QD/ MXene aerogel 1.0 M KPF 6 EC:PC = 1:1 vol% CMC 47.7 521 (X = 106) 3.2 246 [111]
3D macroporous Sb/C 5.0 M KFSI DME CMC 76.2 342 (X = 260) 6.4 176 [112]
Nanoporous Sb 0.8 M KPF 6 EC:DEC = 1:1 vol% CMC 71.0 318 (X = 50) 0.5 265 [12]
Sb/CSN 4.0 M KTFSI EC:DEC = 1:1 vol% Alginate 61.0 551 (X = 100) 0.2 504 [69]
Sb/C/rGO 0.8 M KFSI EC:DEC = 1:1 vol% CMC 46.3 310 (X = 100) 1.5 110 [70]
Sb/NSF-C 5.0 M KFSI DME CMC 55.1 363 (X = 200) 1.0 287 [113]
Sb/CNS 1.0 M KPF EC:DMC = 1:1 vol% CMC 48.0 247 (X = 600) 2.0 101 [114]
6
Sb/C PNFs 1.0 M KPF 6 EC:DEC = 1:1 vol% PMMA 71.3 264 (X = 500) 5.0 208 [115]
BiSb/C 5.0 M KFSI DME Alginate 70.2 320 (X = 600) 2.0 152 [116]
Sb 0.25 Bi 0.75 /C 3.0 M KFSI DME PVDF 45.0 302 (X = 500) 0.5 276 [117]
Layered Sn-Sb 4.0 M KFSI EMC CMC 68.0 296 (X = 150) 5.0 118 [118]
Sb/rGO 0.8 M KPF 6 EC:DEC = 1:1 vol% CMC 49.3 300 (X = 40) 0.5 210 [119]
Sb-based ASSLIB anodes
Although solid electrolytes provide significant advantages in terms of rigidity, thermal stability, and nonflammability, the direct utilization of Li-metal anodes
can induce dendrite formation resulting in short circuits, which remains a critical challenge for ASSLIB development . To address this issue, alloy-based
[122]
anodes that do not form dendrites have been considered. Lewis et al. investigated the critical differences in the SEI layer formation dynamics of LIBs and
ASSLIBs, emphasizing the potential benefits of alloy anodes . Due to its immobility, the solid electrolyte in an ASSLIB inhibits excessive SEI layer formation
[123]
and subsequent electrolyte depletion during continuous cycling. Therefore, alloy-based anodes form mechanically and chemically denser and more stable SEI
layers compared to those of LIBs.