Page 57 - Read Online
P. 57
Page 18 of 30 Yoon et al. Energy Mater 2024;4:400063 https://dx.doi.org/10.20517/energymater.2023.146
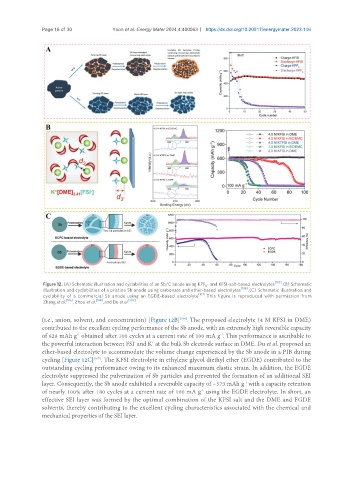
Figure 12. (A) Schematic illustration and cyclabilities of an Sb/C anode using KPF - and KFSI-salt-based electrolytes [105] . (B) Schematic
6
illustration and cyclabilities of a pristine Sb anode using carbonate and ether-based electrolytes [106] . (C) Schematic illustration and
cyclability of a commercial Sb anode using an EGDE-based electrolyte [107] . This figure is reproduced with permission from
Zhang et al. [105] , Zhou et al. [106] , and Du et al. [107] .
(i.e., anion, solvent, and concentration) [Figure 12B] . The proposed electrolyte (4 M KFSI in DME)
[106]
contributed to the excellent cycling performance of the Sb anode, with an extremely high reversible capacity
-1
-1
of 628 mAh g obtained after 100 cycles at a current rate of 100 mA g . This performance is ascribable to
-
+
the powerful interaction between FSI and K at the bulk Sb electrode surface in DME. Du et al. proposed an
ether-based electrolyte to accommodate the volume change experienced by the Sb anode in a PIB during
[107]
cycling [Figure 12C] . The KFSI electrolyte in ethylene glycol diethyl ether (EGDE) contributed to the
outstanding cycling performance owing to its enhanced maximum elastic strain. In addition, the EGDE
electrolyte suppressed the pulverization of Sb particles and prevented the formation of an additional SEI
layer. Consequently, the Sb anode exhibited a reversible capacity of ~573 mAh g with a capacity retention
-1
-1
of nearly 100% after 180 cycles at a current rate of 100 mA g using the EGDE electrolyte. In short, an
effective SEI layer was formed by the optimal combination of the KFSI salt and the DME and EGDE
solvents, thereby contributing to the excellent cycling characteristics associated with the chemical and
mechanical properties of the SEI layer.