Page 46 - Read Online
P. 46
Yoon et al. Energy Mater 2024;4:400063 https://dx.doi.org/10.20517/energymater.2023.146 Page 7 of 30
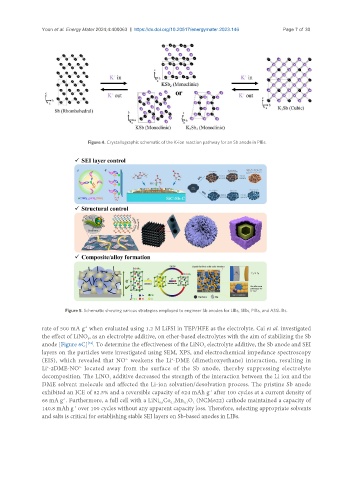
Figure 4. Crystallographic schematic of the K-ion reaction pathway for an Sb anode in PIBs.
Figure 5. Schematic showing various strategies employed to engineer Sb anodes for LIBs, SIBs, PIBs, and ASSLIBs.
rate of 500 mA g when evaluated using 1.2 M LiFSI in TEP/HFE as the electrolyte. Cai et al. investigated
-1
the effect of LiNO , as an electrolyte additive, on ether-based electrolytes with the aim of stabilizing the Sb
3
anode [Figure 6C] . To determine the effectiveness of the LiNO electrolyte additive, the Sb anode and SEI
[74]
3
layers on the particles were investigated using SEM, XPS, and electrochemical impedance spectroscopy
(EIS), which revealed that NO weakens the Li -DME (dimethoxyethane) interaction, resulting in
+
3-
Li -2DME-NO located away from the surface of the Sb anode, thereby suppressing electrolyte
3-
+
decomposition. The LiNO additive decreased the strength of the interaction between the Li ion and the
3
DME solvent molecule and affected the Li-ion solvation/desolvation process. The pristine Sb anode
exhibited an ICE of 82.5% and a reversible capacity of 624 mAh g after 100 cycles at a current density of
-1
66 mA g . Furthermore, a full cell with a LiNi Co Mn O (NCM622) cathode maintained a capacity of
-1
0.2
0.2
2
0.6
140.8 mAh g over 100 cycles without any apparent capacity loss. Therefore, selecting appropriate solvents
-1
and salts is critical for establishing stable SEI layers on Sb-based anodes in LIBs.