Page 44 - Read Online
P. 44
Yoon et al. Energy Mater 2024;4:400063 https://dx.doi.org/10.20517/energymater.2023.146 Page 5 of 30
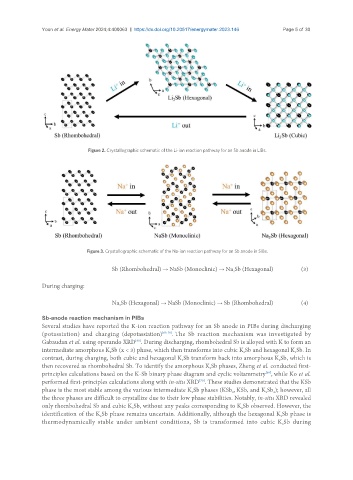
Figure 2. Crystallographic schematic of the Li-ion reaction pathway for an Sb anode in LIBs.
Figure 3. Crystallographic schematic of the Na-ion reaction pathway for an Sb anode in SIBs.
Sb (Rhombohedral) → NaSb (Monoclinic) → Na Sb (Hexagonal) (3)
3
During charging:
Na Sb (Hexagonal) → NaSb (Monoclinic) → Sb (Rhombohedral) (4)
3
Sb-anode reaction mechanism in PIBs
Several studies have reported the K-ion reaction pathway for an Sb anode in PIBs during discharging
(potassiation) and charging (depotassiation) [68-70] . The Sb reaction mechanism was investigated by
Gabaudan et al. using operando XRD . During discharging, rhombohedral Sb is alloyed with K to form an
[68]
intermediate amorphous K Sb (x < 3) phase, which then transforms into cubic K Sb and hexagonal K Sb. In
x
3
3
contrast, during charging, both cubic and hexagonal K Sb transform back into amorphous K Sb, which is
3
x
then recovered as rhombohedral Sb. To identify the amorphous K Sb phases, Zheng et al. conducted first-
x
principles calculations based on the K-Sb binary phase diagram and cyclic voltammetry , while Ko et al.
[69]
performed first-principles calculations along with in-situ XRD . These studies demonstrated that the KSb
[70]
phase is the most stable among the various intermediate K Sb phases (KSb , KSb, and K Sb ); however, all
4
5
2
x
the three phases are difficult to crystallize due to their low phase stabilities. Notably, in-situ XRD revealed
only rhombohedral Sb and cubic K Sb, without any peaks corresponding to K Sb observed. However, the
3
x
identification of the K Sb phase remains uncertain. Additionally, although the hexagonal K Sb phase is
3
x
thermodynamically stable under ambient conditions, Sb is transformed into cubic K Sb during
3