Page 10 - Read Online
P. 10
Page 8 of 14 Shen et al. Soft Sci 2023;3:20 https://dx.doi.org/10.20517/ss.2023.10
Figure 5. UV-vis absorption spectra of PBED, P(BED-T), P(BED-TT) films by spray-spin coating polymerization method. P(BED-TT)
films. BED-T: 2,5-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)thiophene; BED-TT: 2,5-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-
yl)thieno[3,2-b]thiophene.
-1
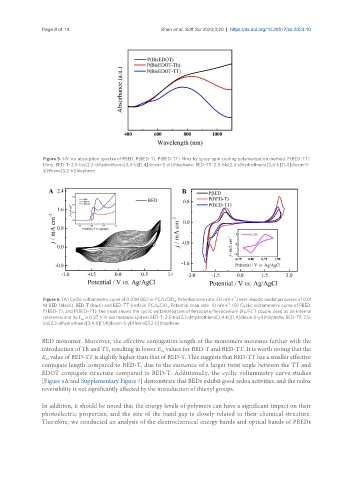
Figure 6. (A) Cyclic voltammetry curve of 0.01M BED in PC/LiClO . Potential scan rate: 50 mV·s . Inset: Anodic oxidation curves of 0.01
4
-1
M BED (black), BED-T (blue) and BED-TT (red) in PC/LiClO . Potential scan rate: 10 mV·s ; (B) Cyclic voltammetry curve of PBED,
4
+
P(BED-T), and P(BED-TT); the inset shows the cyclic voltammogram of ferrocene/ferrocenium (Fc/Fc ) couple used as an internal
reference, and its E is 0.27 V in our measure system.BED-T: 2,5-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)thiophene; BED-TT: 2,5-
ox
bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)thieno[3,2-b]thiophene.
BED monomer. Moreover, the effective conjugation length of the monomers increases further with the
introduction of Th and TT, resulting in lower E values for BED-T and BED-TT. It is worth noting that the
ox
E value of BED-TT is slightly higher than that of BED-T. This suggests that BED-TT has a smaller effective
ox
conjugate length compared to BED-T, due to the existence of a larger twist angle between the TT and
EDOT conjugate structure compared to BED-T. Additionally, the cyclic voltammetry curve studies
[Figure 6A and Supplementary Figure 7] demonstrate that BEDs exhibit good redox activities, and the redox
reversibility is not significantly affected by the introduction of thienyl groups.
In addition, it should be noted that the energy levels of polymers can have a significant impact on their
photoelectric properties, and the size of the band gap is closely related to their chemical structure.
Therefore, we conducted an analysis of the electrochemical energy bands and optical bands of PBEDs