Page 9 - Read Online
P. 9
Shen et al. Soft Sci 2023;3:20 https://dx.doi.org/10.20517/ss.2023.10 Page 7 of 14
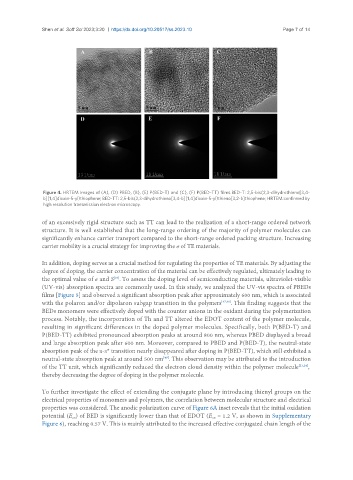
Figure 4. HRTEM images of (A), (D) PBED, (B), (E) P(BED-T) and (C), (F) P(BED-TT) films.BED-T: 2,5-bis(2,3-dihydrothieno[3,4-
b][1,4]dioxin-5-yl)thiophene; BED-TT: 2,5-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)thieno[3,2-b]thiophene; HRTEM:confirmed by
high resolution transmission electron microscopy.
of an excessively rigid structure such as TT can lead to the realization of a short-range ordered network
structure. It is well established that the long-range ordering of the majority of polymer molecules can
significantly enhance carrier transport compared to the short-range ordered packing structure. Increasing
carrier mobility is a crucial strategy for improving the σ of TE materials.
In addition, doping serves as a crucial method for regulating the properties of TE materials. By adjusting the
degree of doping, the carrier concentration of the material can be effectively regulated, ultimately leading to
the optimal value of σ and S . To assess the doping level of semiconducting materials, ultraviolet-visible
[29]
(UV-vis) absorption spectra are commonly used. In this study, we analyzed the UV-vis spectra of PBEDs
films [Figure 5] and observed a significant absorption peak after approximately 600 nm, which is associated
with the polaron and/or dipolaron subgap transition in the polymers [47,48] . This finding suggests that the
BEDs monomers were effectively doped with the counter anions in the oxidant during the polymerization
process. Notably, the incorporation of Th and TT altered the EDOT content of the polymer molecule,
resulting in significant differences in the doped polymer molecules. Specifically, both P(BED-T) and
P(BED-TT) exhibited pronounced absorption peaks at around 800 nm, whereas PBED displayed a broad
and large absorption peak after 600 nm. Moreover, compared to PBED and P(BED-T), the neutral-state
absorption peak of the π-π* transition nearly disappeared after doping in P(BED-TT), which still exhibited a
neutral-state absorption peak at around 500 nm . This observation may be attributed to the introduction
[49]
of the TT unit, which significantly reduced the electron cloud density within the polymer molecule [23,24] ,
thereby decreasing the degree of doping in the polymer molecule.
To further investigate the effect of extending the conjugate plane by introducing thienyl groups on the
electrical properties of monomers and polymers, the correlation between molecular structure and electrical
properties was considered. The anodic polarization curve of Figure 6A inset reveals that the initial oxidation
potential (E ) of BED is significantly lower than that of EDOT (E = 1.2 V, as shown in Supplementary
ox
ox
Figure 6), reaching 0.57 V. This is mainly attributed to the increased effective conjugated chain length of the