Page 228 - Read Online
P. 228
Figueroa-Hall et al. TLR4-mediated signaling in CHME-5 cells
Negative control Unstimulated LPS (1 μg/mL) genes between primary human microglia and human
A microglial cell lines, including CHME-5 cells [26] .
Knowing what we do now about the non-human
Brightfield [25]
origin of CHME-5 cells from Garcia-Mesa et al.
2017, the inability to detect gene expression might
B have been due to the use of human primers. Taking
this into consideration, we characterized TLR4
TLR4
neuroinflammatory signaling in CHME-5 cells, as a rat
cell line, validated that these cells are not of human
C origin, and demonstrated that CHME-5 cells remain
a viable tool to study microglial-like inflammatory
CD68
responses.
D Brightfield imaging revealed morphological
characteristics of “resting” vs. “activated” microglia.
Merge w/DAPI Unstimulated and LPS-treated cells displayed
morphological signatures of microglia, as seen
E in previous studies [22,27-29] , which included smaller
bodies and elongated processes, or amoeboid-
3D
reconstruction shaped and rounder cellular bodies, respectively. This
demonstrates that CHME-5 cells retain characteristics
F that define their role as microglial cells.
3D
side view Microglia up-regulate several activation markers in
response to damage, disease, or loss of homeostatic
conditions, such as CD68, which is a microglial
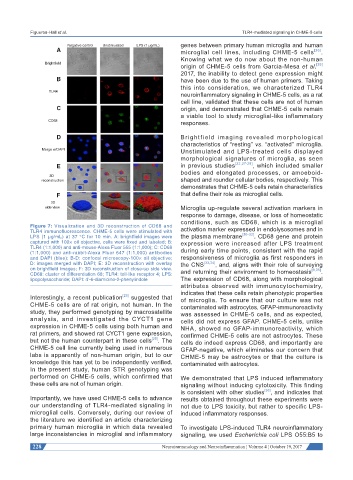
Figure 7: Visualization and 3D reconstruction of CD68 and activation marker expressed in endolysosomes and in
TLR4 immunofluorescence. CHME-5 cells were stimulated with [30-32]
LPS (1 μg/mL) at 37 °C for 10 min. A: brightfield images were the plasma membrane . CD68 gene and protein
captured with 100× oil objective, cells were fixed and labeled; B: expression were increased after LPS treatment
TLR4 (1:1,000) and anti-mouse-Alexa Fluor 555 (1:1,000); C: CD68 during early time points, consistent with the rapid
(1:1,000) and anti-rabbit-Alexa Fluor 647 (1:1,000) antibodies
and DAPI (blue); B-D: confocal microscopy-100× oil objective; responsiveness of microglia as first responders in
D: images merged with DAPI; E: 3D reconstruction with overlay the CNS [33,34] , and, aligns with their role of surveying
on brightfield images; F: 3D reconstruction of close-up side view. and returning their environment to homeostasis [9,35] .
CD68: cluster of differentiation 68; TLR4: toll-like receptor 4; LPS:
lipopolysaccharide; DAPI: 4'-6-diamidino-2-phenylindole The expression of CD68, along with morphological
attributes observed with immunocytochemistry,
indicates that these cells retain phenotypic properties
Interestingly, a recent publication [25] suggested that of microglia. To ensure that our culture was not
CHME-5 cells are of rat origin, not human. In the contaminated with astrocytes, GFAP-immunoreactivity
study, they performed genotyping by macrosatellite was assessed in CHME-5 cells, and as expected,
analysis, and investigated the CYCT1 gene cells did not express GFAP. CHME-5 cells, unlike
expression in CHME-5 cells using both human and NHA, showed no GFAP-immunoreactivity, which
rat primers, and showed rat CYCT1 gene expression, confirmed CHME-5 cells are not astrocytes. These
but not the human counterpart in these cells [25] . The cells do indeed express CD68, and importantly are
CHME-5 cell line currently being used in numerous GFAP-negative, which eliminates our concern that
labs is apparently of non-human origin, but to our CHME-5 may be astrocytes or that the culture is
knowledge this has yet to be independently verified. contaminated with astrocytes.
In the present study, human STR genotyping was
performed on CHME-5 cells, which confirmed that We demonstrated that LPS induced inflammatory
these cells are not of human origin. signaling without inducing cytotoxicity. This finding
is consistent with other studies [36] , and indicates that
Importantly, we have used CHME-5 cells to advance results obtained throughout these experiments were
our understanding of TLR4-mediated signaling in not due to LPS toxicity, but rather to specific LPS-
microglial cells. Conversely, during our review of induced inflammatory responses.
the literature we identified an article characterizing
primary human microglia in which data revealed To investigate LPS-induced TLR4 neuroinflammatory
large inconsistencies in microglial and inflammatory signaling, we used Escherichia coli LPS O55:B5 to
228 Neuroimmunology and Neuroinflammation ¦ Volume 4 ¦ October 19, 2017