Page 48 - Read Online
P. 48
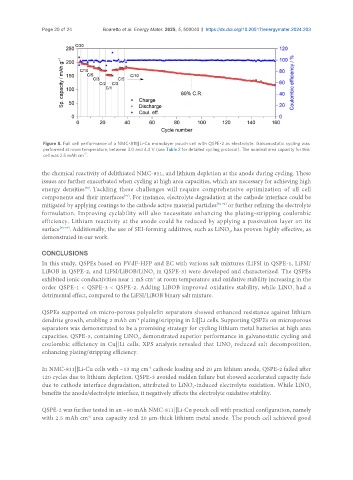
Page 20 of 24 Boaretto et al. Energy Mater. 2025, 5, 500040 https://dx.doi.org/10.20517/energymater.2024.203
Figure 8. Full cell performance of a NMC-811||Li-Cu monolayer pouch cell with QSPE-2 as electrolyte. Galvanostatic cycling was
performed at room temperature, between 3.0 and 4.3 V (see Table 2 for detailed cycling protocol). The nominal area capacity for this
-2
cell was 2.5 mAh cm .
the chemical reactivity of delithiated NMC-811, and lithium depletion at the anode during cycling. These
issues are further exacerbated when cycling at high area capacities, which are necessary for achieving high
energy densities . Tackling these challenges will require comprehensive optimization of all cell
[70]
components and their interfaces . For instance, electrolyte degradation at the cathode interface could be
[71]
mitigated by applying coatings to the cathode active material particles [72-74] or further refining the electrolyte
formulation. Improving cyclability will also necessitate enhancing the plating-stripping coulombic
efficiency. Lithium reactivity at the anode could be reduced by applying a passivation layer on its
surface [75-77] . Additionally, the use of SEI-forming additives, such as LiNO , has proven highly effective, as
3
demonstrated in our work.
CONCLUSIONS
In this study, QSPEs based on PVdF-HFP and EC with various salt mixtures (LiFSI in QSPE-1, LiFSI/
LiBOB in QSPE-2, and LiFSI/LiBOB/LiNO in QSPE-3) were developed and characterized. The QSPEs
3
-1
exhibited ionic conductivities near 1 mS cm at room temperature and oxidative stability increasing in the
order QSPE-1 < QSPE-3 < QSPE-2. Adding LiBOB improved oxidative stability, while LiNO had a
3
detrimental effect, compared to the LiFSI/LiBOB binary salt mixture.
QSPEs supported on micro-porous polyolefin separators showed enhanced resistance against lithium
dendrite growth, enabling 2 mAh cm plating/stripping in Li||Li cells. Supporting QSPEs on microporous
-2
separators was demonstrated to be a promising strategy for cycling lithium metal batteries at high area
capacities. QSPE-3, containing LiNO , demonstrated superior performance in galvanostatic cycling and
3
coulombic efficiency in Cu||Li cells. XPS analysis revealed that LiNO reduced salt decomposition,
3
enhancing plating/stripping efficiency.
-2
In NMC-811||Li-Cu cells with ~13 mg cm cathode loading and 20 µm lithium anode, QSPE-2 failed after
120 cycles due to lithium depletion. QSPE-3 avoided sudden failure but showed accelerated capacity fade
due to cathode interface degradation, attributed to LiNO -induced electrolyte oxidation. While LiNO
3
3
benefits the anode/electrolyte interface, it negatively affects the electrolyte oxidative stability.
QSPE-2 was further tested in an ~80 mAh NMC-811||Li-Cu pouch cell with practical configuration, namely
-2
with 2.5 mAh cm area capacity and 20 µm-thick lithium metal anode. The pouch cell achieved good