Page 46 - Read Online
P. 46
Page 18 of 24 Boaretto et al. Energy Mater. 2025, 5, 500040 https://dx.doi.org/10.20517/energymater.2024.203
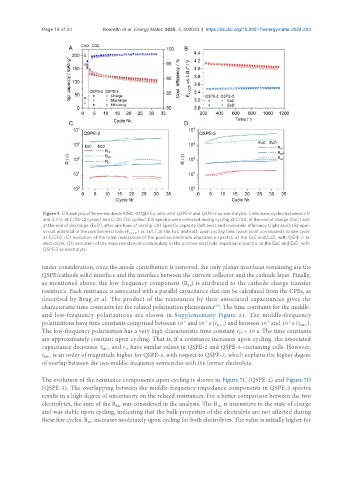
Figure 7. EIS analysis of three-electrode NMC-811||Li-Cu cells with QSPE-2 and QSPE-3 as electrolytes. Cells were cycled between 3.0
and 4.3 V, at C/50 (2 cycles) and C/20 (30 cycles). EIS spectra were collected during cycling at C/20, at the end of charge (EoC) and
at the end of discharge (EoD), after one hour of resting. (A) Specific capacity (left axis) and coulombic efficiency (right axis); (B) open
+
circuit potential of the positive electrode (E +,OCP ) vs. Li/Li , at the EoC and EoD, upon cycling time (each point corresponds to one cycle
at C/20); (C) evolution of the main resistances of the positive electrode impedance spectra, at the EoC and EoD, with QSPE-2 as
electrolyte; (D) evolution of the main resistances contributing to the positive electrode impedance spectra, at the EoC and EoD, with
QSPE-3 as electrolyte.
under consideration, once the anode contribution is removed, the only planar interfaces remaining are the
QSPE/cathode solid interface and the interface between the current collector and the cathode layer. Finally,
as mentioned above, the low-frequency component (R ) is attributed to the cathode charge transfer
LF
resistance. Each resistance is associated with a parallel capacitance that can be calculated from the CPEs, as
described by Brug et al. The product of the resistances by their associated capacitances gives the
characteristic time constants for the related polarization phenomena . The time constants for the middle-
[69]
and low-frequency polarizations are shown in Supplementary Figure 21. The middle-frequency
polarizations have time constants comprised between 10 and 10 s (τ ) and between 10 and 10 s (τ ).
-4
-6
-3
-2
MF,1
MF,2
The low-frequency polarization has a very high characteristic time constant τ > 10 s. The time constants
LF
are approximately constant upon cycling. That is, if a resistance increases upon cycling, the associated
capacitance decreases. τ and τ have similar values in QSPE-2 and QSPE-3-containing cells. However,
LF
MF,2
τ is an order of magnitude higher for QSPE-3, with respect to QSPE-2, which explains the higher degree
MF,1
of overlap between the two middle-frequency semicircles with the former electrolyte.
The evolution of the resistance components upon cycling is shown in Figure 7C (QSPE-2) and Figure 7D
(QSPE-3). The overlapping between the middle-frequency impedance components in QSPE-3 spectra
results in a high degree of uncertainty on the related resistances. For a better comparison between the two
electrolytes, the sum of the R was considered in the analysis. The R is insensitive to the state of charge
HF
MF
and was stable upon cycling, indicating that the bulk properties of the electrolyte are not affected during
these few cycles. R increases moderately upon cycling for both electrolytes. The value is initially higher for
MF