Page 179 - Read Online
P. 179
Li et al. Energy Mater 2023;3:300021 https://dx.doi.org/10.20517/energymater.2023.09 Page 11 of 16
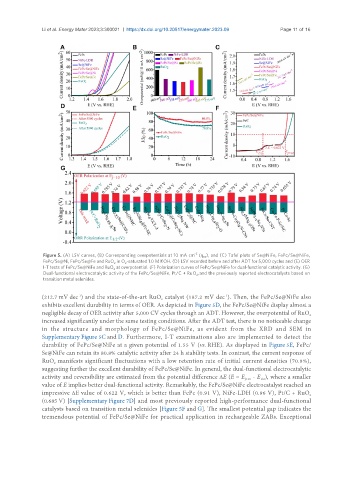
-2
Figure 5. (A) LSV curves, (B) Corresponding overpotentials at 10 mA cm (η ), and (C) Tafel plots of Se@NiFe, FePc/Se@NiFe,
10
FePc/Se@Ni, FePc/Se@Fe and RuO in O -saturated 1.0 M KOH. (D) LSV recorded before and after ADT for 5,000 cycles and (E) OER
2 2
I-T tests of FePc/Se@NiFe and RuO at overpotential. (F) Polarization curves of FePc/Se@NiFe for dual-functional catalytic activity. (G)
2
Dual-functional electrocatalytic activity of the FePc/Se@NiFe, Pt/C + RuO , and the previously reported electrocatalysts based on
2
transition metal selenides.
(212.7 mV dec ) and the state-of-the-art RuO catalyst (187.2 mV dec ). Then, the FePc/Se@NiFe also
-1
-1
2
exhibits excellent durability in terms of OER. As depicted in Figure 5D, the FePc/Se@NiFe display almost a
negligible decay of OER activity after 5,000 CV cycles through an ADT. However, the overpotential of RuO
2
increased significantly under the same testing conditions. After the ADT test, there is no noticeable change
in the structure and morphology of FePc/Se@NiFe, as evident from the XRD and SEM in
Supplementary Figure 5C and D. Furthermore, I-T examinations also are implemented to detect the
durability of FePc/Se@NiFe at a given potential of 1.55 V (vs. RHE). As displayed in Figure 5E, FePc/
Se@NiFe can retain its 80.8% catalytic activity after 24 h stability tests. In contrast, the current response of
RuO manifests significant fluctuations with a low retention rate of initial current densities (70.8%),
2
suggesting further the excellent durability of FePc/Se@NiFe. In general, the dual-functional electrocatalytic
activity and reversibility are estimated from the potential difference ΔE (E = E - E ), where a smaller
j=10
1/2
value of E implies better dual-functional activity. Remarkably, the FePc/Se@NiFe electrocatalyst reached an
impressive ΔE value of 0.622 V, which is better than FePc (0.91 V), NiFe-LDH (0.96 V), Pt/C + RuO
2
(0.685 V) [Supplementary Figure 7D] and most previously reported high-performance dual-functional
catalysts based on transition metal selenides [Figure 5F and G]. The smallest potential gap indicates the
tremendous potential of FePc/Se@NiFe for practical application in rechargeable ZABs. Exceptional