Page 180 - Read Online
P. 180
Page 12 of 16 Li et al. Energy Mater 2023;3:300021 https://dx.doi.org/10.20517/energymater.2023.09
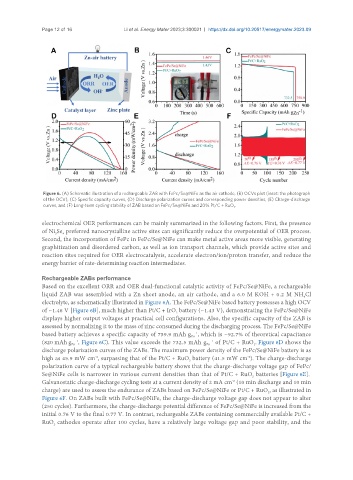
Figure 6. (A) Schematic illustration of a rechargeable ZAB with FePc/Se@NiFe as the air cathode, (B) OCVs plot (inset: the photograph
of the OCV), (C) Specific capacity curves, (D) Discharge polarization curves and corresponding power densities, (E) Charge-discharge
curves, and (F) Long-term cycling stability of ZAB based on FePc/Se@NiFe and 20% Pt/C + RuO .
2
electrochemical OER performances can be mainly summarized in the following factors. First, the presence
of Ni Se preferred nanocrystalline active sites can significantly reduce the overpotential of OER process.
4
3
Second, the incorporation of FePc in FePc/Se@NiFe can make metal active areas more visible, generating
graphitization and disordered carbon, as well as ion transport channels, which provide active sites and
reaction sites required for OER electrocatalysis, accelerate electron/ion/proton transfer, and reduce the
energy barrier of rate-determining reaction intermediates.
Rechargeable ZABs performance
Based on the excellent ORR and OER dual-functional catalytic activity of FePc/Se@NiFe, a rechargeable
liquid ZAB was assembled with a Zn sheet anode, an air cathode, and a 6.0 M KOH + 0.2 M NH Cl
4
electrolyte, as schematically illustrated in Figure 6A. The FePc/Se@NiFe based battery possesses a high OCV
of ~1.46 V [Figure 6B], much higher than Pt/C + IrO battery (~1.43 V), demonstrating the FePc/Se@NiFe
2
displays higher output voltages at practical cell configurations. Also, the specific capacity of the ZAB is
assessed by normalizing it to the mass of zinc consumed during the discharging process. The FePc/Se@NiFe
based battery achieves a specific capacity of 759.9 mAh g , which is ~92.7% of theoretical capacitance
-1
Zn
(820 mAh g , Figure 6C). This value exceeds the 732.5 mAh g of Pt/C + RuO . Figure 6D shows the
-1
-1
2
Zn
Zn
discharge polarization curves of the ZABs. The maximum power density of the FePc/Se@NiFe battery is as
high as 45.9 mW cm , surpassing that of the Pt/C + RuO battery (41.5 mW cm ). The charge-discharge
-2
-2
2
polarization curve of a typical rechargeable battery shows that the charge-discharge voltage gap of FePc/
Se@NiFe cells is narrower in various current densities than that of Pt/C + RuO batteries [Figure 6E].
2
Galvanostatic charge-discharge cycling tests at a current density of 2 mA cm (10 min discharge and 10 min
-2
charge) are used to assess the endurance of ZABs based on FePc/Se@NiFe or Pt/C + RuO , as illustrated in
2
Figure 6F. On ZABs built with FePc/Se@NiFe, the charge-discharge voltage gap does not appear to alter
(250 cycles). Furthermore, the charge-discharge potential difference of FePc/Se@NiFe is increased from the
initial 0.76 V to the final 0.77 V. In contrast, rechargeable ZABs containing commercially available Pt/C +
RuO cathodes operate after 100 cycles, have a relatively large voltage gap and poor stability, and the
2