Page 205 - Read Online
P. 205
Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30 Page 17 of 30
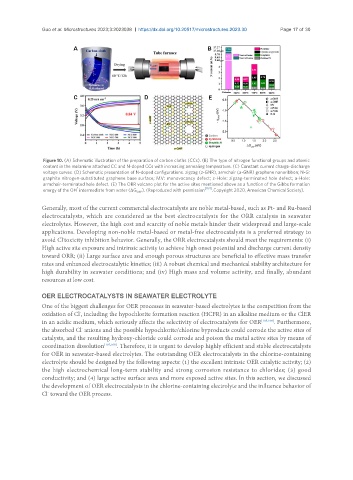
Figure 10. (A) Schematic illustration of the preparation of carbon cloths (CCs). (B) The type of nitrogen functional groups and atomic
content in the melamine attached CC and N-doped CCs with increasing annealing temperature. (C) Constant current charge-discharge
voltage curves. (D) Schematic presentation of N-doped configurations. zigzag (z-GNR), armchair (a-GNR) graphene nanoribbon; N-G:
graphite nitrogen-substituted graphene base surface; MV: monovacancy defect; z-Hole: zigzag-terminated hole defect; a-Hole:
armchair-terminated hole defect. (E) The ORR volcano plot for the active sites mentioned above as a function of the Gibbs formation
*
energy of the OH intermediate from water (ΔG OH* ). (Reproduced with permission [104] . Copyright 2020, American Chemical Society).
Generally, most of the current commercial electrocatalysts are noble metal-based, such as Pt- and Ru-based
electrocatalysts, which are considered as the best electrocatalysts for the ORR catalysis in seawater
electrolytes. However, the high cost and scarcity of noble metals hinder their widespread and large-scale
applications. Developing non-noble metal-based or metal-free electrocatalysts is a preferred strategy to
avoid Cl toxicity inhibition behavior. Generally, the ORR electrocatalysts should meet the requirements: (i)
-
High active site exposure and intrinsic activity to achieve high onset potential and discharge current density
toward ORR; (ii) Large surface area and enough porous structures are beneficial to effective mass transfer
rates and enhanced electrocatalytic kinetics; (iii) A robust chemical and mechanical stability architecture for
high durability in seawater conditions; and (iv) High mass and volume activity, and finally, abundant
resources at low cost.
OER ELECTROCATALYSTS IN SEAWATER ELECTROLYTE
One of the biggest challenges for OER processes in seawater-based electrolytes is the competition from the
oxidation of Cl , including the hypochlorite formation reaction (HCFR) in an alkaline medium or the ClER
-
in an acidic medium, which seriously affects the selectivity of electrocatalysts for OER [105,106] . Furthermore,
the absorbed Cl anions and the possible hypochlorite/chlorine byproducts could corrode the active sites of
-
catalysts, and the resulting hydroxy-chloride could corrode and poison the metal active sites by means of
coordination dissolution [107,108] . Therefore, it is urgent to develop highly efficient and stable electrocatalysts
for OER in seawater-based electrolytes. The outstanding OER electrocatalysts in the chlorine-containing
electrolyte should be designed by the following aspects: (1) the excellent intrinsic OER catalytic activity; (2)
the high electrochemical long-term stability and strong corrosion resistance to chlorides; (3) good
conductivity; and (4) large active surface area and more exposed active sites. In this section, we discussed
the development of OER electrocatalysts in the chlorine-containing electrolyte and the influence behavior of
Cl toward the OER process.
-