Page 203 - Read Online
P. 203
Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30 Page 15 of 30
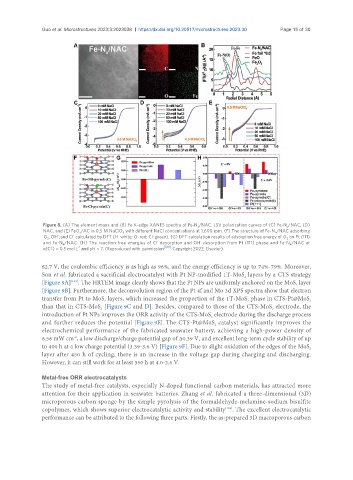
Figure 8. (A) The element maps and (B) Fe K-edge XANES spectra of Fe-N /NAC. LSV polarization curves of (C) Fe-N /NAC, (D)
x
x
NAC, and (E) FeO /AC in 0.5 M NaClO with different NaCl concentrations at 1,600 rpm. (F) The structure of Fe-N /NAC adsorbing
x
4
4
-
-
O , OH , and Cl calculated by DFT (H: white; O: red; Cl: green). (G) DFT calculation results of adsorption free energy of O on Pt (111)
2
2
-
-
and Fe-N /NAC. (H) The reaction-free energies of Cl desorption and OH desorption from Pt (111) phase and Fe-N /NAC at
4 4
-
-1
α(Cl ) = 0.5 mol L and pH = 7. (Reproduced with permission [100] . Copyright 2022, Elsevier).
≤2.7 V, the coulombic efficiency is as high as 96%, and the energy efficiency is up to 74%-79%. Moreover,
Son et al. fabricated a sacrificial electrocatalyst with Pt NP-modified 1T-MoS layers by a CTS strategy
2
[102]
[Figure 9A] . The HRTEM image clearly shows that the Pt NPs are uniformly anchored on the MoS layer
2
[Figure 9B]. Furthermore, the deconvolution region of the Pt 4f and Mo 3d XPS spectra show that electron
transfer from Pt to MoS layers, which increased the proportion of the 1T-MoS phase in CTS-Pt@MoS
2
2
2
than that in CTS-MoS [Figure 9C and D]. Besides, compared to those of the CTS-MoS electrode, the
2
2
introduction of Pt NPs improves the ORR activity of the CTS-MoS electrode during the discharge process
2
and further reduces the potential [Figure 9E]. The CTS-Pt@MoS catalyst significantly improves the
2
electrochemical performance of the fabricated seawater battery, achieving a high-power density of
-2
6.56 mW cm , a low discharge/charge potential gap of Δ0.39 V, and excellent long-term cycle stability of up
to 400 h at a low charge potential (3.39-3.6 V) [Figure 9F]. Due to slight oxidation of the edges of the MoS
2
layer after 450 h of cycling, there is an increase in the voltage gap during charging and discharging.
However, it can still work for at least 350 h at 4.0-2.6 V.
Metal-free ORR electrocatalysts
The study of metal-free catalysts, especially N-doped functional carbon materials, has attracted more
attention for their application in seawater batteries. Zhang et al. fabricated a three-dimensional (3D)
microporous carbon sponge by the simple pyrolysis of the formaldehyde-melamine-sodium bisulfite
copolymer, which shows superior electrocatalytic activity and stability . The excellent electrocatalytic
[103]
performance can be attributed to the following three parts. Firstly, the as-prepared 3D macroporous carbon