Page 202 - Read Online
P. 202
Page 14 of 30 Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30
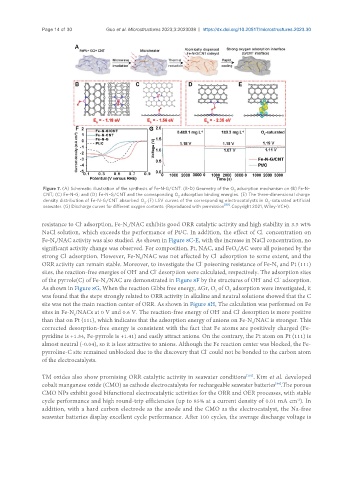
Figure 7. (A) Schematic illustration of the synthesis of Fe-N-G/CNT. (B-D) Geometry of the O adsorption mechanism on (B) Fe-N-
2
CNT; (C) Fe-N-G; and (D) Fe-N-G/CNT and the corresponding O adsorption binding energies. (E) The three-dimensional charge
2
density distribution of Fe-N-G/CNT absorbed O . (F) LSV curves of the corresponding electrocatalysts in O -saturated artificial
2
2
seawater. (G) Discharge curves for different oxygen contents. (Reproduced with permission [99] . Copyright 2021, Wiley-VCH).
resistance to Cl adsorption, Fe-N /NAC exhibits good ORR catalytic activity and high stability in 3.5 wt%
-
x
NaCl solution, which exceeds the performance of Pt/C. In addition, the effect of Cl concentration on
-
Fe-N /NAC activity was also studied. As shown in Figure 8C-E, with the increase in NaCl concentration, no
x
significant activity change was observed. For composition, Pt, NAC, and FeO /AC were all poisoned by the
x
-
strong Cl adsorption. However, Fe-N /NAC was not affected by Cl adsorption to some extent, and the
-
x
-
ORR activity can remain stable. Moreover, to investigate the Cl poisoning resistance of Fe-N and Pt (111)
x
sites, the reaction-free energies of OH and Cl desorption were calculated, respectively. The adsorption sites
-
-
-
-
of the pyrrole(C) of Fe-N /NAC are demonstrated in Figure 8F by the structures of OH and Cl adsorption.
4
As shown in Figure 8G, When the reaction Gibbs free energy, ΔGr, O of O adsorption were investigated, it
2
2
was found that the steps strongly related to ORR activity in alkaline and neutral solutions showed that the C
site was not the main reaction center of ORR. As shown in Figure 8H, The calculation was performed on Fe
-
sites in Fe-N /NACs at 0 V and 0.6 V. The reaction-free energy of OH and Cl desorption is more positive
-
x
than that on Pt (111), which indicates that the adsorption energy of anions on Fe-N /NAC is stronger. This
4
corrected desorption-free energy is consistent with the fact that Fe atoms are positively charged (Fe-
pyridine is +1.34, Fe-pyrrole is +1.41) and easily attract anions. On the contrary, the Pt atom on Pt (111) is
almost neutral (-0.04), so it is less attractive to anions. Although the Fe reaction center was blocked, the Fe-
-
pyrroline-C site remained unblocked due to the discovery that Cl could not be bonded to the carbon atom
of the electrocatalysts.
TM oxides also show promising ORR catalytic activity in seawater conditions . Kim et al. developed
[101]
cobalt manganese oxide (CMO) as cathode electrocatalysts for rechargeable seawater batteries .The porous
[84]
CMO NPs exhibit good bifunctional electrocatalytic activities for the ORR and OER processes, with stable
cycle performance and high round-trip efficiencies (up to 85% at a current density of 0.01 mA cm ). In
-2
addition, with a hard carbon electrode as the anode and the CMO as the electrocatalyst, the Na-free
seawater batteries display excellent cycle performance. After 100 cycles, the average discharge voltage is