Page 199 - Read Online
P. 199
Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30 Page 11 of 30
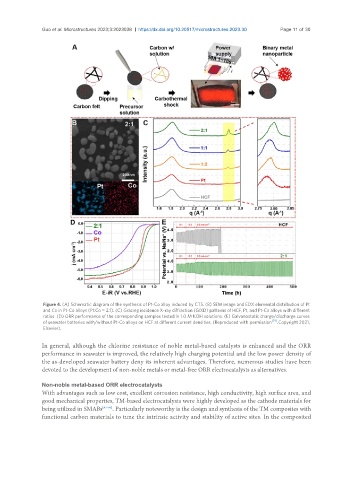
Figure 4. (A) Schematic diagram of the synthesis of Pt-Co alloy induced by CTS. (B) SEM image and EDX elemental distribution of Pt
and Co in Pt-Co alloys (Pt:Co = 2:1). (C) Grazing incidence X-ray diffraction (GIXD) patterns of HCF, Pt, and Pt-Co alloys with different
ratios. (D) ORR performance of the corresponding samples tested in 1.0 M KOH solutions. (E) Galvanostatic charge/discharge curves
of seawater batteries with/without Pt-Co alloys on HCF at different current densities. (Reproduced with permission [91] . Copyright 2021,
Elsevier).
In general, although the chlorine resistance of noble metal-based catalysts is enhanced and the ORR
performance in seawater is improved, the relatively high charging potential and the low power density of
the as-developed seawater battery deny its inherent advantages. Therefore, numerous studies have been
devoted to the development of non-noble metals or metal-free ORR electrocatalysts as alternatives.
Non-noble metal-based ORR electrocatalysts
With advantages such as low cost, excellent corrosion resistance, high conductivity, high surface area, and
good mechanical properties, TM-based electrocatalysts were highly developed as the cathode materials for
being utilized in SMABs [93-96] . Particularly noteworthy is the design and synthesis of the TM composites with
functional carbon materials to tune the intrinsic activity and stability of active sites. In the composited