Page 195 - Read Online
P. 195
Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30 Page 7 of 30
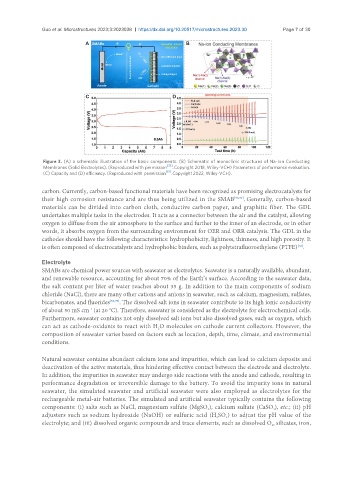
Figure 3. (A) a schematic illustration of the basic components. (B) Schematic of monoclinic structures of Na-Ion Conducting
[33]
Membranes (Solid Electrolytes). (Reproduced with permission . Copyright 2018, Wiley-VCH) Parameters of performance evaluation.
[61]
(C) Capacity and (D) efficiency. (Reproduced with permission . Copyright 2022, Wiley-VCH).
carbon. Currently, carbon-based functional materials have been recognized as promising electrocatalysts for
their high corrosion resistance and are thus being utilized in the SMAB [70,71] . Generally, carbon-based
materials can be divided into carbon cloth, conductive carbon paper, and graphitic fiber. The GDL
undertakes multiple tasks in the electrodes. It acts as a connector between the air and the catalyst, allowing
oxygen to diffuse from the air atmosphere to the surface and further to the inner of an electrode, or in other
words, it absorbs oxygen from the surrounding environment for OER and ORR catalysis. The GDL in the
cathodes should have the following characteristics: hydrophobicity, lightness, thinness, and high porosity. It
is often composed of electrocatalysts and hydrophobic binders, such as polytetrafluoroethylene (PTFE) .
[72]
Electrolyte
SMABs are chemical power sources with seawater as electrolytes. Seawater is a naturally available, abundant,
and renewable resource, accounting for about 70% of the Earth’s surface. According to the seawater data,
the salt content per liter of water reaches about 35 g. In addition to the main components of sodium
chloride (NaCl), there are many other cations and anions in seawater, such as calcium, magnesium, sulfates,
bicarbonates, and fluorides [53,73] . The dissolved salt ions in seawater contribute to its high ionic conductivity
-1
o
of about 50 mS cm (at 20 C). Therefore, seawater is considered as the electrolyte for electrochemical cells.
Furthermore, seawater contains not only dissolved salt ions but also dissolved gases, such as oxygen, which
can act as cathode-oxidants to react with H O molecules on cathode current collectors. However, the
2
composition of seawater varies based on factors such as location, depth, time, climate, and environmental
conditions.
Natural seawater contains abundant calcium ions and impurities, which can lead to calcium deposits and
deactivation of the active materials, thus hindering effective contact between the electrode and electrolyte.
In addition, the impurities in seawater may undergo side reactions with the anode and cathode, resulting in
performance degradation or irreversible damage to the battery. To avoid the impurity ions in natural
seawater, the simulated seawater and artificial seawater were also employed as electrolytes for the
rechargeable metal-air batteries. The simulated and artificial seawater typically contains the following
components: (i) salts such as NaCl, magnesium sulfate (MgSO ), calcium sulfate (CaSO ), etc.; (ii) pH
4
4
adjusters such as sodium hydroxide (NaOH) or sulfuric acid (H SO ) to adjust the pH value of the
4
2
electrolyte; and (iii) dissolved organic compounds and trace elements, such as dissolved O , silicates, iron,
2