Page 193 - Read Online
P. 193
Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30 Page 5 of 30
However, due to the large amount of consumption of precious silver and the resulting high cost, the SAB
was only being used in the military area. Recently, many efforts have been devoted to reducing the battery
manufacturing cost and to further increase the total power. For instance, a variety of Mg, Al, zinc (Zn), and
[34]
Na alloys have been explored for being used as the anodes [35-48] , while the cuprous chloride , cuprous
iodide [49,50] , lead chloride and mercurous chloride have been explored as the cathodes of SABs. However,
[51]
[52]
up to now, none of these systems can completely replace the Mg/AgCl system.
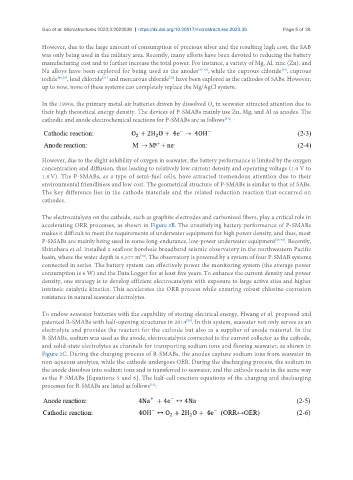
In the 1990s, the primary metal-air batteries driven by dissolved O in seawater attracted attention due to
2
their high theoretical energy density. The devices of P-SMABs mainly use Zn, Mg, and Al as anodes. The
cathodic and anode electrochemical reactions for P-SMABs are as follows :
[53]
However, due to the slight solubility of oxygen in seawater, the battery performance is limited by the oxygen
concentration and diffusion, thus leading to relatively low current density and operating voltage (1.0 V to
1.8 V). The P-SMABs, as a type of semi-fuel cells, have attracted tremendous attention due to their
environmental friendliness and low cost. The geometrical structure of P-SMABs is similar to that of SABs.
The key difference lies in the cathode materials and the related reduction reaction that occurred on
cathodes.
The electrocatalysts on the cathode, such as graphite electrodes and carbonized fibers, play a critical role in
accelerating ORR processes, as shown in Figure 2B. The unsatisfying battery performance of P-SMABs
makes it difficult to meet the requirements of underwater equipment for high power density, and thus, most
P-SMABs are mainly being used in some long-endurance, low-power underwater equipment [53-55] . Recently,
Shinohara et al. installed a seafloor borehole broadband seismic observatory in the northwestern Pacific
basin, where the water depth is 5,577 m . The observatory is powered by a system of four P-SMAB systems
[54]
connected in series. The battery system can effectively power the monitoring system (the average power
consumption is 6 W) and the Data Logger for at least five years. To enhance the current density and power
density, one strategy is to develop efficient electrocatalysts with exposure to large active sites and higher
intrinsic catalytic kinetics. This accelerates the ORR process while ensuring robust chlorine-corrosion
resistance in natural seawater electrolytes.
To endow seawater batteries with the capability of storing electrical energy, Hwang et al. proposed and
patented R-SMABs with half-opening structures in 2014 . In this system, seawater not only serves as an
[33]
electrolyte and provides the reactant for the cathode but also as a supplier of anode material. In the
R-SMABs, sodium was used as the anode, electrocatalysts connected to the current collector as the cathode,
and solid-state electrolytes as channels for transporting sodium ions and flowing seawater, as shown in
Figure 2C. During the charging process of R-SMABs, the anodes capture sodium ions from seawater in
non-aqueous anolytes, while the cathode undergoes OER. During the discharging process, the sodium in
the anode dissolves into sodium ions and is transferred to seawater, and the cathode reacts in the same way
as the P-SMABs [Equations 5 and 6]. The half-cell reaction equations of the charging and discharging
processes for R-SMABs are listed as follows :
[51]