Page 210 - Read Online
P. 210
Page 22 of 30 Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30
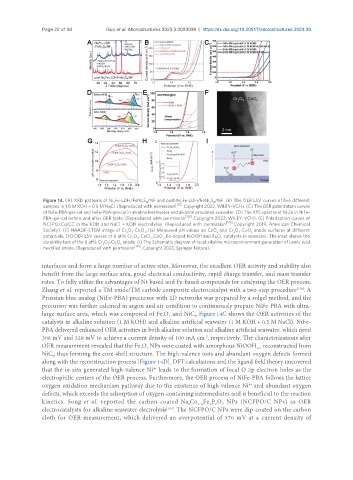
Figure 14. (A) XRD patterns of Ni Fe-LDH/FeNi S /NF and post-Ni Fe-LDH/FeNi S /NF. (B) The OER LSV curves of five different
2 4
2
2
2 4
samples in 1.0 M KOH + 0.5 M NaCl. (Reproduced with permission [127] . Copyright 2022, WILEY-VCH). (C) The OER polarization curves
of NiFe-PBA-gel-cal and FeFe-PBA-gel-cal in alkaline freshwater and alkaline simulated seawater. (D) The XPS spectra of Ni 2p in NiFe-
PBA-gel-cal before and after OER tests. (Reproduced with permission [128] . Copyright 2022, WILEY-VCH). (E) Polarization curves of
NCFPO/C@CC in the KOH and NaCl + KOH electrolytes. (Reproduced with permission [129] . Copyright 2019, American Chemical
Society). (F) HAADF-STEM image of Cr O -CoO . (G) Measured pH values on CoO and Cr O -CoO anode surfaces at different
2
2
x
3
x
x
3
potentials. (H) OER LSV curves of 6 at% Cr O -CoO , CoO , Fe-doped NiOOH and RuO catalysts in seawater. The inset shows the
x
3
2
x
2
durability test of the 6 at% Cr O -CoO anode. (I) The Schematic diagram of local alkaline microenvironment generation of Lewis acid
2
x
3
modified anode. (Reproduced with permission [130] . Copyright 2023, Springer Nature).
interfaces and form a large number of active sites. Moreover, the excellent OER activity and stability also
benefit from the large surface area, good electrical conductivity, rapid charge transfer, and mass transfer
rates. To fully utilize the advantages of Ni-based and Fe-based compounds for catalyzing the OER process,
Zhang et al. reported a TM oxide/TM carbide composite electrocatalyst with a two-step procedure . A
[128]
Prussian blue analog (NiFe-PBA) precursor with 2D networks was prepared by a solgel method, and the
precursor was further calcined in argon and air condition to continuously prepare NiFe-PBA with ultra-
large surface area, which was composed of Fe O and NiC . Figure 14C shows the OER activities of the
3
4
x
catalysts in alkaline solution (1 M KOH) and alkaline artificial seawater (1 M KOH + 0.5 M NaCl). NiFe-
PBA delivered enhanced OER activities in both alkaline solution and alkaline artificial seawater, which need
-2
308 mV and 329 mV to achieve a current density of 100 mA cm , respectively. The characterizations after
OER measurement revealed that the Fe O NPs were coated with amorphous NiOOH reconstructed from
2-x
3
4
NiC , thus forming the core-shell structure. The high-valence ions and abundant oxygen defects formed
x
along with the reconstruction process [Figure 14D]. DFT calculations and the ligand field theory uncovered
4+
that the in-situ generated high-valence Ni leads to the formation of local O 2p electron holes as the
electrophilic centers of the OER process. Furthermore, the OER process of NiFe-PBA follows the lattice
oxygen oxidation mechanism pathway due to the existence of high valence Ni and abundant oxygen
4+
defects, which exceeds the adsorption of oxygen-containing intermediates and is beneficial to the reaction
kinetics. Song et al. reported the carbon-coated Na Co Fe P O NPs (NCFPO/C NPs) as OER
7
1-x
2
x 2
[129]
electrocatalysts for alkaline seawater electrolysis . The NCFPO/C NPs were dip-coated on the carbon
cloth for OER measurement, which delivered an overpotential of 370 mV at a current density of