Page 209 - Read Online
P. 209
Guo et al. Microstructures 2023;3:2023038 https://dx.doi.org/10.20517/microstructures.2023.30 Page 21 of 30
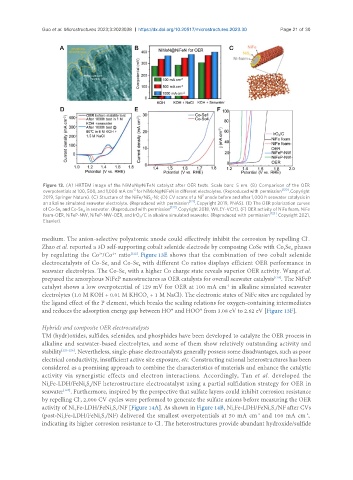
Figure 13. (A) HRTEM image of the NiMoN@NiFeN catalyst after OER tests. Scale bars: 5 nm. (B) Comparison of the OER
-2 [120]
overpotentials at 100, 500, and 1,000 mA cm for NiMoN@NiFeN in different electrolytes. (Reproduced with permission . Copyright
3
2019, Springer Nature). (C) Structure of the NiFe/NiS -Ni; (D) CV scans of a Ni anode before and after 1,000 h seawater catalysis in
x
an alkaline simulated seawater electrolyte. (Reproduced with permission [121] . Copyright 2019, PNAS). (E) The OER polarization curves
of Co-Se and Co-Se in seawater. (Reproduced with permission [122] . Copyright 2018, WILEY-VCH). (F) OER activity of NiFe foam, NiFe
1
4
foam-OER, NiFeP-NW, NiFeP-NW-OER, and IrO /C in alkaline simulated seawater. (Reproduced with permission [123] . Copyright 2021,
2
Elsevier).
medium. The anion-selective polyatomic anode could effectively inhibit the corrosion by repelling Cl .
-
Zhao et al. reported a 3D self-supporting cobalt selenide electrode by composing CoSe with Co Se phases
8
9
by regulating the Co /Co ratio . Figure 13E shows that the combination of two cobalt selenide
[122]
2+
3+
electrocatalysts of Co-Se and Co-Se with different Co ratios displays efficient OER performance in
1
4
seawater electrolytes. The Co-Se with a higher Co charge state reveals superior OER activity. Wang et al.
1
[119]
prepared the amorphous NiFeP nanostructures as OER catalysts for overall seawater catalysis . The NiFeP
catalyst shows a low overpotential of 129 mV for OER at 100 mA cm in alkaline simulated seawater
-2
electrolytes (1.0 M KOH + 0.01 M KHCO + 1 M NaCl). The electronic states of NiFe sites are regulated by
3
the ligand effect of the P element, which breaks the scaling relations for oxygen-containing intermediates
and reduces the adsorption energy gap between HO* and HOO* from 3.08 eV to 2.62 eV [Figure 13F].
Hybrids and composite OER electrocatalysts
TM (hydr)oxides, sulfides, selenides, and phosphides have been developed to catalyze the OER process in
alkaline and seawater-based electrolytes, and some of them show relatively outstanding activity and
stability [123-126] . Nevertheless, single-phase electrocatalysts generally possess some disadvantages, such as poor
electrical conductivity, insufficient active site exposure, etc. Constructing rational heterostructures has been
considered as a promising approach to combine the characteristics of materials and enhance the catalytic
activity via synergistic effects and electron interactions. Accordingly, Tan et al. developed the
Ni Fe-LDH/FeNi S /NF heterostructure electrocatalyst using a partial sulfidation strategy for OER in
2 4
2
seawater . Furthermore, inspired by the perspective that sulfate layers could inhibit corrosion resistance
[127]
-
by repelling Cl , 2,000 CV cycles were performed to generate the sulfate anions before measuring the OER
activity of Ni Fe-LDH/FeNi S /NF [Figure 14A]. As shown in Figure 14B, Ni Fe-LDH/FeNi S /NF after CVs
2
2 4
2 4
2
-2
(post-Ni Fe-LDH/FeNi S /NF) delivered the smallest overpotentials at 50 mA cm and 100 mA cm ,
-2
2 4
2
indicating its higher corrosion resistance to Cl . The heterostructures provide abundant hydroxide/sulfide
-