Page 111 - Read Online
P. 111
Page 14 of 19 Hussain et al. Soft Sci. 2025, 5, 21 https://dx.doi.org/10.20517/ss.2025.02
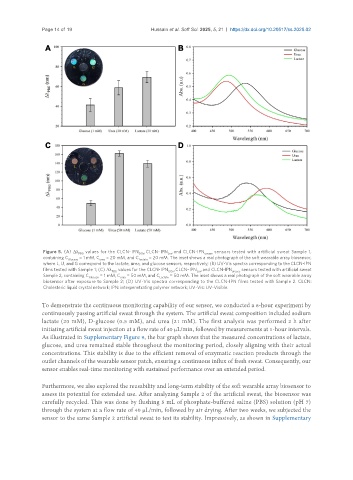
Figure 5. (A) Δλ values for the CLCN-IPN , CLCN-IPN , and CLCN-IPN sensors tested with artificial sweat Sample 1,
PBG GOx Lox urease
containing C = 1 mM, C = 20 mM, and C = 20 mM. The inset shows a real photograph of the soft wearable array biosensor,
Glucose urea Lactate
where L, U, and G correspond to the lactate, urea, and glucose sensors, respectively; (B) UV-Vis spectra corresponding to the CLCN-IPN
films tested with Sample 1; (C) Δλ values for the CLCN-IPN , CLCN-IPN , and CLCN-IPN sensors tested with artificial sweat
PBG GOx Lox urease
Sample 2, containing C = 1 mM, C = 50 mM, and C = 50 mM. The inset shows a real photograph of the soft wearable array
Glucose urea Lactate
biosensor after exposure to Sample 2; (D) UV-Vis spectra corresponding to the CLCN-IPN films tested with Sample 2. CLCN:
Cholesteric liquid crystal network; IPN: interpenetrating polymer network; UV-Vis: UV-Visible.
To demonstrate the continuous monitoring capability of our sensor, we conducted a 6-hour experiment by
continuously passing artificial sweat through the system. The artificial sweat composition included sodium
lactate (20 mM), D-glucose (0.5 mM), and urea (21 mM). The first analysis was performed 2 h after
initiating artificial sweat injection at a flow rate of 40 µL/min, followed by measurements at 1-hour intervals.
As illustrated in Supplementary Figure 9, the bar graph shows that the measured concentrations of lactate,
glucose, and urea remained stable throughout the monitoring period, closely aligning with their actual
concentrations. This stability is due to the efficient removal of enzymatic reaction products through the
outlet channels of the wearable sensor patch, ensuring a continuous influx of fresh sweat. Consequently, our
sensor enables real-time monitoring with sustained performance over an extended period.
Furthermore, we also explored the reusability and long-term stability of the soft wearable array biosensor to
assess its potential for extended use. After analyzing Sample 2 of the artificial sweat, the biosensor was
carefully recycled. This was done by flushing 5 mL of phosphate-buffered saline (PBS) solution (pH 7)
through the system at a flow rate of 40 µL/min, followed by air drying. After two weeks, we subjected the
sensor to the same Sample 2 artificial sweat to test its stability. Impressively, as shown in Supplementary