Page 111 - Read Online
P. 111
Hochhalter et al. Association between HCMV and GBM
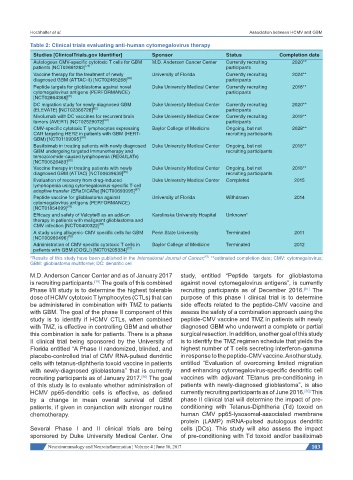
Table 2: Clinical trials evaluating anti-human cytomegalovirus therapy
Studies [ClinicalTrials.gov Identifier] Sponsor Status Completion date
Autologous CMV-specific cytotoxic T cells for GBM M.D. Anderson Cancer Center Currently recruiting 2020**
patients [NCT02661282] [79] participants
Vaccine therapy for the treatment of newly University of Florida Currently recruiting 2024**
diagnosed GBM (ATTAC-II) [NCT02465268] [80] participants
Peptide targets for glioblastoma against novel Duke University Medical Center Currently recruiting 2018**
cytomegalovirus antigens (PERFORMANCE) participants
[NCT02864368] [81]
DC migration study for newly-diagnosed GBM Duke University Medical Center Currently recruiting 2020**
(ELEVATE) [NCT02366728] [82] participants
Nivolumab with DC vaccines for recurrent brain Duke University Medical Center Currently recruiting 2019**
tumors (AVERT) [NCT02529072] [83] participants
CMV-specific cytotoxic T lymphocytes expressing Baylor College of Medicine Ongoing, but not 2028**
CAR targeting HER2 in patients with GBM (HERT- recruiting participants
GBM) [NCT01109095] [84]
Basiliximab in treating patients with newly diagnosed Duke University Medical Center Ongoing, but not 2018**
GBM undergoing targeted immunotherapy and recruiting participants
temozolomide-caused lymphopenia (REGULATe)
[NCT00626483] [85]
Vaccine therapy in treating patients with newly Duke University Medical Center Ongoing, but not 2018**
diagnosed GBM (ATTAC) [NCT00639639] [86] recruiting participants
Evaluation of recovery from drug-induced Duke University Medical Center Completed 2015
lymphopenia using cytomegalovirus-specific T-cell
adoptive transfer (ERaDICATe) [NCT00693095] [87]
Peptide vaccine for glioblastoma against University of Florida Withdrawn 2014
cytomegalovirus antigens (PERFORMANCE)
[NCT01854099] [78]
Efficacy and safety of Valcyte® as an add-on Karolinska University Hospital Unknown*
therapy in patients with malignant glioblastoma and
CMV infection [NCT00400322] [88]
A study using allogenic-CMV specific cells for GBM Penn State University Terminated 2011
[NCT00990496] [76]
Administration of CMV-specific cytotoxic T cells in Baylor College of Medicine Terminated 2012
patients with GBM (COGLI) [NCT01205334] [77]
[65]
*Results of this study have been published in the International Journal of Cancer; **estimated completion date; CMV: cytomegalovirus;
GBM: glioblastoma multiforme; DC: dendritic cell
M.D. Anderson Cancer Center and as of January 2017 study, entitled “Peptide targets for glioblastoma
is recruiting participants. The goals of this combined against novel cytomegalovirus antigens”, is currently
[79]
Phase I/II study is to determine the highest tolerable recruiting participants as of December 2016. The
[81]
dose of HCMV cytotoxic T lymphocytes (CTLs) that can purpose of this phase I clinical trial is to determine
be administered in combination with TMZ to patients side effects related to the peptide-CMV vaccine and
with GBM. The goal of the phase II component of this assess the safety of a combination approach using the
study is to identify if HCMV CTLs, when combined peptide-CMV vaccine and TMZ in patients with newly
with TMZ, is effective in controlling GBM and whether diagnosed GBM who underwent a complete or partial
this combination is safe for patients. There is a phase surgical resection. In addition, another goal of this study
II clinical trial being sponsored by the University of is to identify the TMZ regimen schedule that yields the
Florida entitled “A Phase II randomized, blinded, and highest number of T cells secreting interferon-gamma
placebo-controlled trial of CMV RNA-pulsed dendritic in response to the peptide-CMV vaccine. Another study,
cells with tetanus-diphtheria toxoid vaccine in patients entitled “Evaluation of overcoming limited migration
with newly-diagnosed glioblastoma” that is currently and enhancing cytomegalovirus-specific dendritic cell
recruiting participants as of January 2017. The goal vaccines with adjuvant TEtanus pre-conditioning in
[80]
of this study is to evaluate whether administration of patients with newly-diagnosed glioblastoma”, is also
HCMV pp65-dendritic cells is effective, as defined currently recruiting participants as of June 2016. This
[82]
by a change in mean overall survival of GBM phase II clinical trial will determine the impact of pre-
patients, if given in conjunction with stronger routine conditioning with Tetanus-Diphtheria (Td) toxoid on
chemotherapy. human CMV pp65-lysosomal-associated membrane
protein (LAMP) mRNA-pulsed autologous dendritic
Several Phase I and II clinical trials are being cells (DCs). This study will also assess the impact
sponsored by Duke University Medical Center. One of pre-conditioning with Td toxoid and/or basiliximab
Neuroimmunology and Neuroinflammation ¦ Volume 4 ¦ June 16, 2017 103