Page 43 - Read Online
P. 43
Page 328 Genvigir et al. J Transl Genet Genom 2020;4:320-55 I http://dx.doi.org/10.20517/jtgg.2020.37
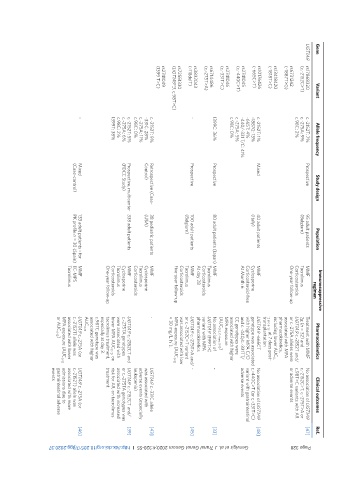
Ref. [47] [48] [33] [45] [43] [39] [46]
Clinical outcomes No association of UGT1A9 c.-2152C>T, c.-275T>A or c.98T>C variants with AR or adverse events No association of UGT1A9 genotype was associated c.-440C>T (or c.-331T>C) variant with gastrointestinal adverse events - - UGT1A9 c.-331C allele was associated with adverse events (especially leukopenia) UGT1A9 c.-2152CT and/ or c.-275TA genotypes was associated with increased risk for AR, on tacrolimus treatment UGT1A9 c.-27
Pharmacokinetics Treatment with MMF 2g (n = 32) and UGT1A9 c.-2152T and/ or c.-275A alleles were associated with MPA pharmacokinetics, including lower AUC 0- 12 and 6-12 , at 7 days post- transplantation UGT1A9 -665CT with higher MPA C/D and c.-440/c.-331TT/ CC genotypes were associated with higher MPA exposure (AUC 0-2, 0-4 and 0-12 ) No association of UGT1A9 I399T>C variant with MPA pharmacokinetics UGT1A9 c.-275T>A and/ o
Immunosuppressive regimen MMF Tacrolimus Corticosteroids One-year follow-up MMF Cyclosporine Corticosteroid-free At Month 6 MMF Tacrolimus Corticosteroids At day 28 MMF Tacrolimus Corticosteroids Five-year follow-up MMF Cyclosporine Tacrolimus Corticosteroids MMF Cyclosporine Tacrolimus Corticosteroids One-year follow-up MMF EC-MPS Tacrolimus
Population 95 adult patients (Belgium) 40 adult patients (Italy) 80 adult patients (Japan) 100 adult patients (Belgium) 38 pediatric patients (USA) 338 adult patients 133 adult patients - for PK profile n = 30 (Spain)
Study design Prospective Mixed Prospective Prospective Retrospective (Case- Control) Prospective, multicenter (FDCC Study) Mixed (Case-control)
Allele frequency c.-2152T: 7% c.-275A: 9% c.98C: 2% c.-2152T: 1% -1887G: 13% -665T: 4% -440/-331 T/C: 41% c.-275A: 5% c.98C: 0% I399C: 36% - c.-2152T: 9% -331C: 28% c.-275A: 11% c.98C: 0% c.-2152T: 5% c.-275A: 6% c.98C: 2% I399T: 38% -
Variant (UGT1A9*3, c.98T>C)
rs17868320 (c.-2152C>T) rs6731242 (-1887T>G) rs13418420 (-1818T>C) rs10176426 (-665C>T) rs2741045 (c.-440C>T) rs2741046 (c.-331T>C) rs6714486 (c.-275T>A) rs3832043 (-118delT) rs72551330 rs2741049 (I399 T>C)
Gene UGT1A9