Page 46 - Read Online
P. 46
Genvigir et al. J Transl Genet Genom 2020;4:320-55 I http://dx.doi.org/10.20517/jtgg.2020.37 Page 331
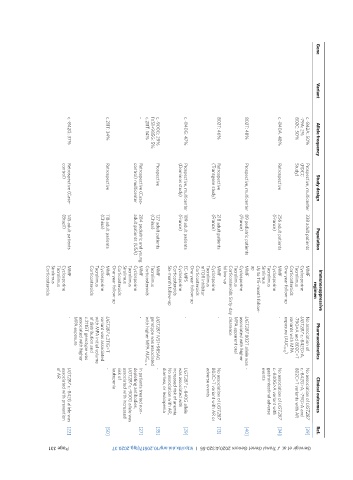
Ref. [39] [34] [40] [13] [29] [35] [27] [50] [22]
Clinical outcomes No association of UGT2B7 c.-842G>A, -79G>A and 802C>T variants with AR No association of UGT2B7 c.-840G>A variant with gastrointestinal adverse events No association of UGT2B7 802C>T variant with AR or adverse events UGT2B7 c.-840G allele was associated with increased risk of anemia. No association with AR, diarrhea, or leukopenia - In patients treated non- depleting antibodies, UGT2B7 c.-900G allele was associated
Pharmacokinetics No association of UGT2B7 c.-842G>A, -79G>A and 802C>T variants with MPA exposure (AUC 0-12 ) - UGT2B7 802T allele was - associated with higher MPA apparent oral - - UGT2B7 IVS1+985AG genotype was associated with higher MPA AUC 0-12 - UGT2B7 c.211G>T variant was associated with MPA initial volume of distribution and c.211GT genotype was associated with higher MPA exposure -
Immunosuppressive regimen MMF Cyclosporine Tacrolimus Corticosteroids One-year follow-up MMF Cyclosporine Tacrolimus Sirolimus Up to 115-month follow- up MMF Cyclosporine Tacrolimus Corticosteroids Sixty-day clearance follow-up MMF Cyclosporine Tacrolimus mTOR inhibitor Corticosteroids One-year follow-up EC-MPS Cyclosporine Corticosteroids Six-month follow-up MMF Tacrolimus Corticosteroids MMF Cyclosporine Tacrolimus Siroli
Population 338 adult patients 256 adult patients (France) 89 pediatric patients (France) 218 adult patients (France) 189 adult patients (France) 127 adult patients (China) 284 pediatric and young adult patients (USA) 118 adult patients (China) 145 adult patients (Brazil)
Study design Prospective, multicenter (FDCC Study) Retrospective Prospective, multicenter Retrospective (Transgene study) Prospective, multicenter (Dominos study) Prospective Retrospective (Case- control) multicenter Retrospective Retrospective (Case- control)
Allele frequency c.-842A: 50% -79A: 2% 802C: 50% c.-840A: 48% 802T: 48% 802T: 46% c.-840G: 47% c.-900G: 29% IVS1+985G: 5% c.211T: 14% - c.211T: 34% c.-842G: 37%
Variant
Gene