Page 41 - Read Online
P. 41
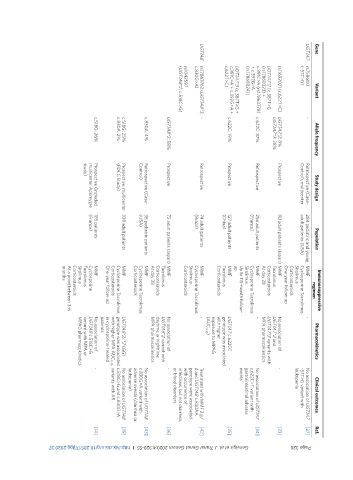
Page 326 Genvigir et al. J Transl Genet Genom 2020;4:320-55 I http://dx.doi.org/10.20517/jtgg.2020.37
Ref. [27] [33] [34] [35] [42] [36] [43] [39] [14]
Clinical outcomes No association of UGT1A7 -57T>G variant with leukopenia - No association of UGT1A7 c.622C>T variant with gastrointestinal adverse events - Treatment with MMF 2 g/ d and UGT1A8 c.830AA genotype were associated with occurrence of infections, but not diarrhea, or blood disorders - No association of UGT1A8 c.830G>A variant with adverse events (diarrhea or leukopenia) No association of UGT1A8 -
Pharmacokinetics No association of UGT1A7*2 and UGT1A7*3 variants with MPA pharmacokinetics UGT1A7 c.622CC genotype was associated exposure to MPAG No association of UGT1A8*2 variant with daytime or nighttime MPA pharmacokinetics UGT1A8*2/*2 (GG) genotype was associated c.518C>G and c.830G>A with higher MPA AUC 0-12 , variants with AR in cyclosporine-treated No association of UGT1A8 c.518C>G variant with MPA or MPAG pharmacoki
- - with higher (AUC 0-12 ) - - patients
Immunosuppressive regimen MMF Cyclosporine Tacrolimus Sirolimus Corticosteroids One-year follow-up MMF Tacrolimus Corticosteroids At day 28 MMF Cyclosporine Tacrolimus Sirolimus Up to 115-month follow- up MMF Tacrolimus Corticosteroids MMF Cyclosporine Tacrolimus Sirolimus Corticosteroids MMF Tacrolimus Corticosteroids At day 28 MMF Cyclosporine Tacrolimus Corticosteroids MMF Cyclosporine Tacrolimus Corticosteroids One-year
284 pediatric and young adult patients (USA) 80 adult patients (Japan) 256 adult patients (France) 127 adult patients (China) 74 adult patients (Brazil) 72 adult patients (Japan) 38 pediatric patients (USA) 338 adult patients 185 patients (France)
Population
Study design Retrospective (Case- Control), multicenter Prospective Retrospective Prospective Retrospective Prospective Retrospective (Case- Control) Prospective, multicenter (FDCC Study) Prospective (included multicenter Apomygre study)
Allele frequency UGT1A7*2: 8% UGT1A7*3: 28% c.622C: 37% c.622C: 19% UGT1A8*2: 58% c.830A: 4% c.518G: 25% c.830A: 2% c.518G: 26%
- -
Variant rs11692021 (c.622T>C) UGT1A7*2 [c.387T>G c.391C>A (rs17863778) UGT1A7*3 (c.387T>G + c.391C>A + c.392G>A + rs17863762 (UGT1A8*3, (UGT1A8*2, c.518C>G)
rs7586110 (-57T>G) (rs17868323) + + c.392G>A (rs17868324)] c.622T>C) c.830G>A) rs1042597
Gene UGT1A7 UGT1A8