Page 40 - Read Online
P. 40
Genvigir et al. J Transl Genet Genom 2020;4:320-55 I http://dx.doi.org/10.20517/jtgg.2020.37 Page 325
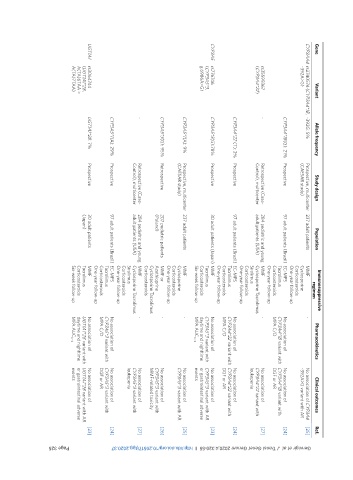
Ref. [25] [24] [27] [24] [23] [25] [26] [27] [24] [23]
Clinical outcomes No association of CYP3A4 -392A>G variant with AR No association of CYP3A4*1B variant with DGF or AR No association of CYP3A4*22 variant with leukopenia No association of DGF or AR No association of CYP3A5*3 variant with AR or gastrointestinal adverse events No association of CYP3A5*3 variant with AR No association of CYP3A5*3 variant with MMF-related toxicity No association of CYP3A5*3 variant with leukopenia
Pharmacokinetics No association of CYP3A4*1B variant with No association of CYP3A4*22 variant with CYP3A4*22 variant with No association of CYP3A5*3 variant with daytime and nighttime No association of CYP3A5*3 variant with No association of UGT1A1*28 variant with daytime and nighttime
- MPA C/D - MPA C/D MPA AUC 0-12 - - - MPA C/D MPA AUC 0-12
Immunosuppressive regimen MMF Cyclosporine Corticosteroids One-year follow-up EC-MPS Tacrolimus Corticosteroids One-year follow-up MMF Cyclosporine Tacrolimus Sirolimus Corticosteroids One-year follow-up EC-MPS Tacrolimus Corticosteroids One-year follow-up MMF Tacrolimus Corticosteroids Six-week follow-up MMF Cyclosporine Corticosteroids One-year follow-up MMF or Azathioprine Cyclosporine Tacrolimus Corticosteroids MMF Cycl
Population 237 adult patients 97 adult patients (Brazil) 284 pediatric and young adult patients (USA) 97 adult patients (Brazil) 30 adult patients (Japan) 237 adult patients 207 pediatric patients (Poland) 284 pediatric and young adult patients (USA) 97 adult patients (Brazil) 30 adult patients (Japan)
Study design Prospective, multicenter (CAESAR study) Prospective Retrospective (Case- Control), multicenter Prospective Prospective Prospective, multicenter (CAESAR study) Retrospective Retrospective (Case- Control), multicenter Prospective Prospective
Allele frequency -392G: 5% CYP3A4*1B(G): 21% - CYP3A4*22 (T): 2% CYP3A5*3(G):78% CYP3A5*1(A): 9% CYP3A5*3(G): 95% - CYP3A5*1(A): 29% UGT1A1*28: 7%
Variant rs2740574 (CYP3A4*1B, -392A>G) rs35599367 (CYP3A4*22) rs776746 (CYP3A5*3, g.6986A>G) rs3064744 (UGT1A1*28, A(TA)6TAA > A(TA)7TAA)
Gene CYP3AA4 CYP3A5 UGT1A1