Page 96 - Read Online
P. 96
Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08 Page 19 of 37
1416.0 0.467 [133] (cal.)
1421.0 0.490 This work(cal.)
L ↔ Fe Y + hcp-Y Eutectic 1173±10 ~0.660 [115] (exp.)
2
1173.0 0.651 [127] (cal.)
1201.0 0.661 [132] (cal.)
1156.0 0.601 [19] (cal.)
1146.0 0.612 [131] (PTD) (cal.)
1118.0 0.637 [131] (ETD) (cal.)
1120.0 0.640 [133] (cal.)
1163.5 0.656 This work(cal.)
L + bcc-Y ↔ hcp-Y Peritectic 1758.0 — [115] (exp.)
1752.0 0.979 [127] (cal.)
1756.0 0.983 [132] (cal.)
1752.0 0.978 [19] (cal.)
1750.0 0.979 [131] (PTD) (cal.)
1749.0 0.976 [131] (ETD) (cal.)
1754.0 0.980 [133] (cal.)
1757.1 0.981 This work(cal.)
[104,105] [96]
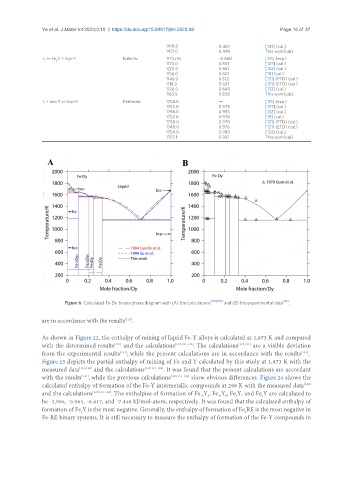
Figure 6. Calculated Fe-Dy binary phase diagram with (A) the calculations and (B) the experimental data .
are in accordance with the results .
[115]
As shown in Figure 22, the enthalpy of mixing of liquid Fe-Y alloys is calculated at 1,873 K and compared
[121]
with the determined results and the calculations [127,131-133] . The calculations [127,131] are a visible deviation
from the experimental results , while the present calculations are in accordance with the results .
[121]
[121]
Figure 23 depicts the partial enthalpy of mixing of Fe and Y calculated by this study at 1,873 K with the
measured data [121,122] and the calculations [127,131-133] . It was found that the present calculations are accordant
[121]
with the results , while the previous calculations [127,131-133] show obvious differences. Figure 24 shows the
calculated enthalpy of formation of the Fe-Y intermetallic compounds at 298 K with the measured data
[123]
and the calculations [127,131-133] . The enthalpies of formation of Fe Y , Fe Y , Fe Y, and Fe Y are calculated to
23 6
2
3
17 2
be -2.994, -5.061, -6.417, and -7.446 kJ/mol-atom, respectively. It was found that the calculated enthalpy of
formation of Fe Y is the most negative. Generally, the enthalpy of formation of Fe RE is the most negative in
2
2
Fe-RE binary systems. It is still necessary to measure the enthalpy of formation of the Fe-Y compounds in