Page 94 - Read Online
P. 94
Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08 Page 17 of 37
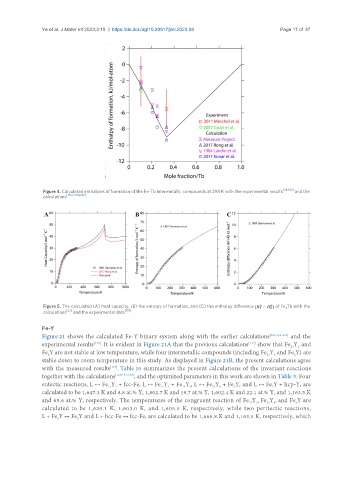
Figure 4. Calculated enthalpies of formation of the Fe-Tb intermetallic compounds at 298 K with the experimental results [54,102] and the
calculations [19,27,104,140] .
Figure 5. The calculated (A) heat capacity, (B) the entropy of formation, and (C) the enthalpy difference of Fe Tb with the
2
calculations [27] and the experimental data [101] .
Fe-Y
Figure 21 shows the calculated Fe-Y binary system along with the earlier calculations [127,131-133] and the
[115]
experimental results . It is evident in Figure 21A that the previous calculations show that Fe Y and
[127]
23 6
Fe Y are not stable at low temperature, while four intermetallic compounds (including Fe Y and Fe Y) are
2
2
23 6
stable down to room temperature in this study. As displayed in Figure 21B, the present calculations agree
with the measured results . Table 10 summarizes the present calculations of the invariant reactions
[115]
together with the calculations [127,131-133] , and the optimized parameters in this work are shown in Table 5. Four
eutectic reactions, L ↔ Fe Y + fcc-Fe, L ↔ Fe Y + Fe Y , L ↔ Fe Y + Fe Y, and L ↔ Fe Y + hcp-Y, are
23 6
2
3
23 6
17 2
17 2
calculated to be 1,647.3 K and 8.8 at.% Y, 1,602.7 K and 19.7 at.% Y, 1,602.4 K and 22.1 at.% Y, and 1,163.5 K
and 65.6 at.% Y, respectively. The temperatures of the congruent reaction of Fe Y , Fe Y , and Fe Y are
3
17 2
23 6
calculated to be 1,620.1 K, 1,603.0 K, and 1,605.5 K, respectively, while two peritectic reactions,
L + Fe Y ↔ Fe Y and L + bcc-Fe ↔ fcc-Fe, are calculated to be 1,666.8 K and 1,163.5 K, respectively, which
2
3