Page 98 - Read Online
P. 98
Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08 Page 21 of 37
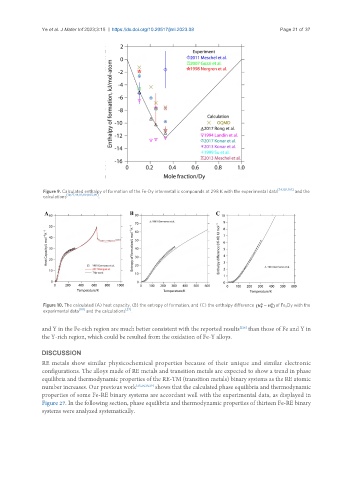
Figure 9. Calculated enthalpy of formation of the Fe-Dy intermetallic compounds at 298 K with the experimental data [54,102,103] and the
calculations [19,27,44,83,104,105,141] .
Figure 10. The calculated (A) heat capacity, (B) the entropy of formation, and (C) the enthalpy difference of Fe Dy with the
2
experimental data [101] and the calculations [27] .
[126]
and Y in the Fe-rich region are much better consistent with the reported results than those of Fe and Y in
the Y-rich region, which could be resulted from the oxidation of Fe-Y alloys.
DISCUSSION
RE metals show similar physicochemical properties because of their unique and similar electronic
configurations. The alloys made of RE metals and transition metals are expected to show a trend in phase
equilibria and thermodynamic properties of the RE-TM (transition metals) binary systems as the RE atomic
number increases. Our previous work [25,26,28,29] shows that the calculated phase equilibria and thermodynamic
properties of some Fe-RE binary systems are accordant well with the experimental data, as displayed in
Figure 27. In the following section, phase equilibria and thermodynamic properties of thirteen Fe-RE binary
systems were analyzed systematically.