Page 101 - Read Online
P. 101
Page 24 of 37 Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08
Figure 14. The calculated (A) heat capacity, (B) the entropy of formation and (C) the enthalpy difference of Fe Er with the
experimental data [101] and the calculated results [110-112] .
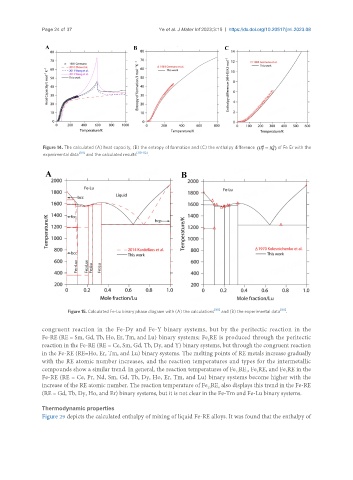
Figure 15. Calculated Fe-Lu binary phase diagram with (A) the calculations [90] and (B) the experimental data [88] .
congruent reaction in the Fe-Dy and Fe-Y binary systems, but by the peritectic reaction in the
Fe-RE (RE = Sm, Gd, Tb, Ho, Er, Tm, and Lu) binary systems; Fe RE is produced through the peritectic
2
reaction in the Fe-RE (RE = Ce, Sm, Gd, Tb, Dy, and Y) binary systems, but through the congruent reaction
in the Fe-RE (RE=Ho, Er, Tm, and Lu) binary systems. The melting points of RE metals increase gradually
with the RE atomic number increases, and the reaction temperatures and types for the intermetallic
compounds show a similar trend. In general, the reaction temperatures of Fe RE , Fe RE, and Fe RE in the
17
2
2
3
Fe-RE (RE = Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, and Lu) binary systems become higher with the
increase of the RE atomic number. The reaction temperature of Fe RE also displays this trend in the Fe-RE
6
23
(RE = Gd, Tb, Dy, Ho, and Er) binary systems, but it is not clear in the Fe-Tm and Fe-Lu binary systems.
Thermodynamic properties
Figure 29 depicts the calculated enthalpy of mixing of liquid Fe-RE alloys. It was found that the enthalpy of