Page 104 - Read Online
P. 104
Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08 Page 27 of 37
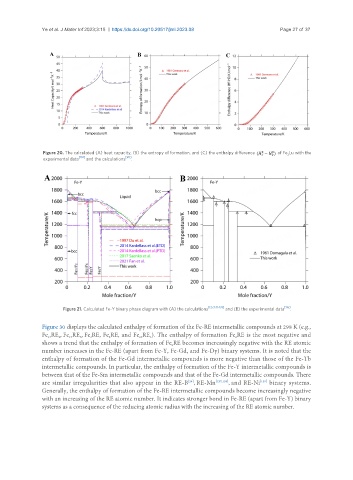
Figure 20. The calculated (A) heat capacity, (B) the entropy of formation, and (C) the enthalpy difference of Fe Lu with the
2
experimental data [101] and the calculations [90] .
Figure 21. Calculated Fe-Y binary phase diagram with (A) the calculations [127,131-133] and (B) the experimental data [115] .
Figure 30 displays the calculated enthalpy of formation of the Fe-RE intermetallic compounds at 298 K (e.g.,
Fe RE , Fe RE , Fe RE, Fe RE, and Fe RE ). The enthalpy of formation Fe RE is the most negative and
5
17
2
6
2
23
3
2
17
shows a trend that the enthalpy of formation of Fe RE becomes increasingly negative with the RE atomic
2
number increases in the Fe-RE (apart from Fe-Y, Fe-Gd, and Fe-Dy) binary systems. It is noted that the
enthalpy of formation of the Fe-Gd intermetallic compounds is more negative than those of the Fe-Tb
intermetallic compounds. In particular, the enthalpy of formation of the Fe-Y intermetallic compounds is
between that of the Fe-Sm intermetallic compounds and that of the Fe-Gd intermetallic compounds. There
[24]
[139]
are similar irregularities that also appear in the RE-B , RE-Mn [137,138] , and RE-Ni binary systems.
Generally, the enthalpy of formation of the Fe-RE intermetallic compounds become increasingly negative
with an increasing of the RE atomic number. It indicates stronger bond in Fe-RE (apart from Fe-Y) binary
systems as a consequence of the reducing atomic radius with the increasing of the RE atomic number.