Page 107 - Read Online
P. 107
Page 30 of 37 Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08
[126]
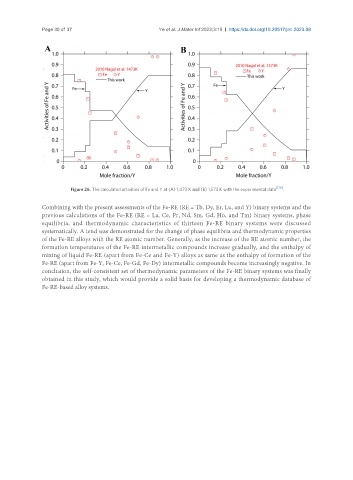
Figure 26. The calculated activities of Fe and Y at (A) 1,473 K and (B) 1,573 K with the experimental data .
Combining with the present assessments of the Fe-RE (RE = Tb, Dy, Er, Lu, and Y) binary systems and the
previous calculations of the Fe-RE (RE = La, Ce, Pr, Nd, Sm, Gd, Ho, and Tm) binary systems, phase
equilibria, and thermodynamic characteristics of thirteen Fe-RE binary systems were discussed
systematically. A tend was demonstrated for the change of phase equilibria and thermodynamic properties
of the Fe-RE alloys with the RE atomic number. Generally, as the increase of the RE atomic number, the
formation temperatures of the Fe-RE intermetallic compounds increase gradually, and the enthalpy of
mixing of liquid Fe-RE (apart from Fe-Ce and Fe-Y) alloys as same as the enthalpy of formation of the
Fe-RE (apart from Fe-Y, Fe-Ce, Fe-Gd, Fe-Dy) intermetallic compounds become increasingly negative. In
conclusion, the self-consistent set of thermodynamic parameters of the Fe-RE binary systems was finally
obtained in this study, which would provide a solid basis for developing a thermodynamic database of
Fe-RE-based alloy systems.