Page 89 - Read Online
P. 89
Page 12 of 37 Ye et al. J Mater Inf 2023;3:15 https://dx.doi.org/10.20517/jmi.2023.08
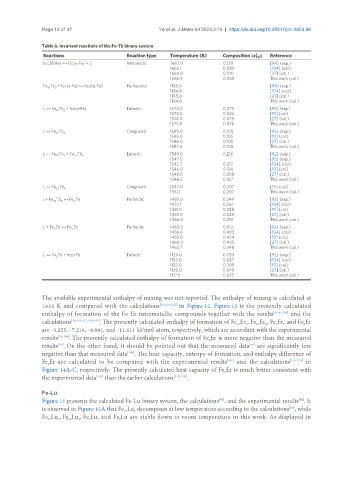
Table 6. Invariant reactions of the Fe-Tb binary system
Reactions Reaction type Temperature (K) Composition () Reference
bcc(δ-Fe) ↔ fcc(γ-Fe) + L Metatectic 1667.0 0.120 [94] (exp.)
1665.1 0.080 [104] (cal.)
1664.0 0.100 [27] (cal.)
1666.9 0.050 This work (cal.)
Fe Tb + fcc(γ-Fe) ↔ bcc(α-Fe) Peritectoid 1185.0 [94] (exp.)
17
2
1184.8 [104] (cal.)
1185.0 [27] (cal.)
1184.8 This work (cal.)
L ↔ Fe Tb + fcc(γ-Fe) Eutectic 1574.0 0.075 [95] (exp.)
17 2
1579.0 0.082 [19] (cal.)
1576.0 0.075 [27] (cal.)
1575.0 0.076 This work (cal.)
L ↔ Fe Tb Congruent 1589.0 0.105 [95] (exp.)
17 2
1585.0 0.105 [19] (cal.)
1586.0 0.105 [27] (cal.)
1587.5 0.105 This work (cal.)
L ↔ Fe Tb + Fe Tb 6 Eutectic 1549.0 0.216 [92] (exp.)
23
17
2
1547.0 [95] (exp.)
1542.7 0.212 [104] (cal.)
1546.0 0.190 [19] (cal.)
1546.0 0.208 [27] (cal.)
1546.5 0.167 This work (cal.)
L ↔ Fe Tb 6 Congruent 1547.0 0.207 [19] (cal.)
23
1561.1 0.207 This work (cal.)
L+ Fe Tb ↔ Fe Tb Peritectic 1485.0 0.349 [92] (exp.)
6
3
23
1473.1 0.361 [104] (cal.)
1481.0 0.388 [19] (cal.)
1485.0 0.340 [27] (cal.)
1486.0 0.319 This work (cal.)
L + Fe Tb ↔ Fe Tb Peritectic 1460.0 0.412 [92] (exp.)
3
2
1456.6 0.403 [104] (cal.)
1459.0 0.424 [19] (cal.)
1460.0 0.405 [27] (cal.)
1462.7 0.348 This work (cal.)
L ↔ Fe Tb + hcp-Tb Eutectic 1120.0 0.720 [92] (exp.)
2
1123.0 0.687 [104] (cal.)
1122.0 0.700 [19] (cal.)
1120.0 0.690 [27] (cal.)
1117.1 0.675 This work (cal.)
The available experimental enthalpy of mixing was not reported. The enthalpy of mixing is calculated at
1833 K and compared with the calculations [19,110-112] in Figure 12. Figure 13 is the presently calculated
enthalpy of formation of the Fe-Er intermetallic compounds together with the results [44,54,103] and the
calculations [19,110-112,140,141] . The presently calculated enthalpy of formation of Fe Er , Fe Er , Fe Er, and Fe Er
2
17
6
23
2
3
are -3.255, -7.216, -8.881, and -11.611 kJ/mol-atom, respectively, which are accordant with the experimental
results [54,103] . The presently calculated enthalpy of formation of Fe Er is more negative than the measured
2
results . On the other hand, it should be pointed out that the measured data are significantly less
[44]
[44]
[103]
negative than that measured data . The heat capacity, entropy of formation, and enthalpy difference of
Fe Er are calculated to be compared with the experimental results and the calculations [110-112] in
[101]
2
Figure 14A-C, respectively. The presently calculated heat capacity of Fe Er is much better consistent with
2
the experimental data than the earlier calculations [110-112] .
[101]
Fe-Lu
Figure 15 presents the calculated Fe-Lu binary system, the calculations , and the experimental results . It
[90]
[88]
is observed in Figure 15A that Fe Lu decomposes at low temperature according to the calculations , while
[90]
2
17
Fe Lu , Fe Lu , Fe Lu, and Fe Lu are stable down to room temperature in this work. As displayed in
23
2
17
2
3
6