Page 142 - Read Online
P. 142
Chong et al. J Mater Inf 2023;3:21 https://dx.doi.org/10.20517/jmi.2023.17 Page 11 of 18
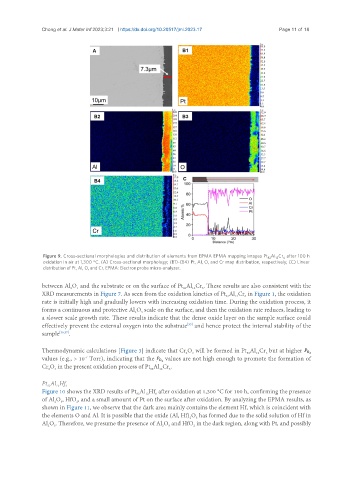
Figure 9. Cross-sectional morphologies and distribution of elements from EPMA EPMA mapping images Pt Al Cr after 100 h
82
12
6
oxidation in air at 1,300 °C. (A) Cross-sectional morphology; (B1)-(B4) Pt, Al, O, and Cr map distribution, respectively; (C) Linear
distribution of Pt, Al, O, and Cr. EPMA: Electron probe micro-analyzer.
between Al O and the substrate or on the surface of Pt Al Cr . These results are also consistent with the
2
12
6
3
82
XRD measurements in Figure 7. As seen from the oxidation kinetics of Pt Al Cr in Figure 1, the oxidation
82
6
12
rate is initially high and gradually lowers with increasing oxidation time. During the oxidation process, it
forms a continuous and protective Al O scale on the surface, and then the oxidation rate reduces, leading to
2
3
a slower scale growth rate. These results indicate that the dense oxide layer on the sample surface could
[35]
effectively prevent the external oxygen into the substrate and hence protect the internal stability of the
sample [36,37] .
Thermodynamic calculations [Figure 3] indicate that Cr O will be formed in Pt Al Cr but at higher P
6
2
o2
82
12
3
values (e.g., > 10 Torr), indicating that the P values are not high enough to promote the formation of
-7
o2
Cr O in the present oxidation process of Pt Al Cr .
12
6
82
3
2
Pt Al Hf 6
12
82
Figure 10 shows the XRD results of Pt Al Hf after oxidation at 1,300 °C for 100 h, confirming the presence
6
82
12
of Al O , HfO , and a small amount of Pt on the surface after oxidation. By analyzing the EPMA results, as
2
3
2
shown in Figure 11, we observe that the dark area mainly contains the element Hf, which is coincident with
the elements O and Al. It is possible that the oxide (Al, Hf) O has formed due to the solid solution of Hf in
2
3
Al O . Therefore, we presume the presence of Al O and HfO in the dark region, along with Pt, and possibly
3
2
2
2
3