Page 145 - Read Online
P. 145
Page 14 of 18 Chong et al. J Mater Inf 2023;3:21 https://dx.doi.org/10.20517/jmi.2023.17
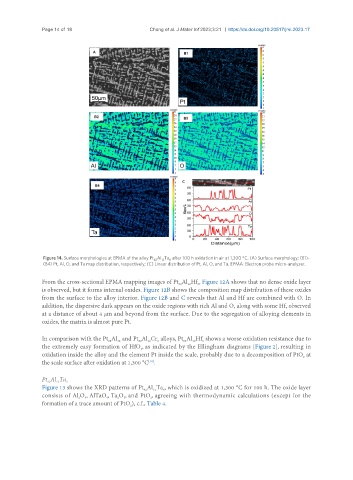
Figure 14. Surface morphologies at EPMA of the alloy Pt Al Ta after 100 h oxidation in air at 1,300 °C. (A) Surface morphology; (B1)-
12
6
82
(B4) Pt, Al, O, and Ta map distribution, respectively; (C) Linear distribution of Pt, Al, O, and Ta. EPMA: Electron probe micro-analyzer.
From the cross-sectional EPMA mapping images of Pt Al Hf , Figure 12A shows that no dense oxide layer
12
82
6
is observed, but it forms internal oxides. Figure 12B shows the composition map distribution of these oxides
from the surface to the alloy interior. Figure 12B and C reveals that Al and Hf are combined with O. In
addition, the dispersive dark appears on the oxide regions with rich Al and O, along with some Hf, observed
at a distance of about 4 μm and beyond from the surface. Due to the segregation of alloying elements in
oxides, the matrix is almost pure Pt.
In comparison with the Pt Al and Pt Al Cr alloys, Pt Al Hf shows a worse oxidation resistance due to
12
82
12
12
82
6
6
88
the extremely easy formation of HfO , as indicated by the Ellingham diagrams [Figure 2], resulting in
2
oxidation inside the alloy and the element Pt inside the scale, probably due to a decomposition of PtO at
2
[10]
the scale surface after oxidation at 1,300 °C .
Pt Al Ta 6
12
82
Figure 13 shows the XRD patterns of Pt Al Ta , which is oxidized at 1,300 °C for 100 h. The oxide layer
6
82
12
consists of Al O , AlTaO , Ta O , and PtO , agreeing with thermodynamic calculations (except for the
4
5
2
3
2
2
formation of a trace amount of PtO ), c.f., Table 4.
2