Page 47 - Read Online
P. 47
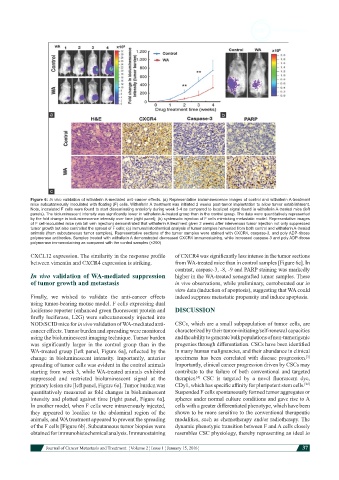
Figure 6: In vivo validation of withaferin A-mediated anti-cancer effects. (a) Representative bioluminescence images of control and withaferin A-treatment
mice subcutaneously inoculated with floating (F) cells. Withaferin A treatment was initiated 2 weeks post-tumor implantation to allow tumor establishment.
Note, inoculated F cells were found to start disseminating anteriorly during week 3-4 as compared to localized signal found in withaferin A-treated mice (left
panels). The bioluminescent intensity was significantly lower in withaferin A-treated group than in the control group. The data were quantitatively represented
by the fold change in bioluminescence intensity over time (right panel); (b) systematic injection of F cells mimicking metastatic model. Representative images
of F cell-inoculated mice (via tail vein injection) demonstrated that withaferin A treatment given 2 weeks after intervenous tumor injection not only suppressed
tumor growth but also controlled the spread of F cells; (c) immunohistochemical analysis of tumor samples harvested from both control and withaferin A-treated
animals (from subcutaneous tumor samples). Representative sections of the tumor samples were stained with CXCR4, caspase-3, and poly ADP-ribose
polymerase antibodies. Samples treated with withaferin A demonstrated decreased CXCR4 immunostaining, while increased caspase-3 and poly ADP-ribose
polymerase immunostaining as compared with the control samples (×200)
CXCL12 expression. The similarity in the response profile of CXCR4 was significantly less intense in the tumor sections
between vimentin and CXCR4 expression is striking. from WA-treated mice than in control samples [Figure 6c]. In
contrast, caspase-3, -8, -9 and PARP staining was markedly
In vivo validation of WA-mediated suppression higher in the WA-treated xenografted tumor samples. These
of tumor growth and metastasis in vivo observations, while preliminary, corroborated our in
vitro data (induction of apoptosis), suggesting that WA could
Finally, we wished to validate the anti-cancer effects indeed suppress metastatic propensity and induce apoptosis.
using tumor-bearing mouse model. F cells expressing dual
luciferase reporter (enhanced green fluorescent protein and DISCUSSION
firefly luciferase, L2G) were subcutaneously injected into
NOD/SCID mice for in vivo validation of WA-mediated anti- CSCs, which are a small subpopulation of tumor cells, are
cancer effects. Tumor burden and spreading were monitored characterized by their tumor-initiating/self-renewal capacities
using the bioluminescent imaging technique. Tumor burden and the ability to generate bulk populations of non-tumorigenic
was significantly larger in the control group than in the progenies through differentiation. CSCs have been identified
WA-treated group [left panel, Figure 6a], reflected by the in many human malignancies, and their abundance in clinical
[3]
change in bioluminescent intensity. Importantly, anterior specimens has been correlated with disease progression.
spreading of tumor cells was evident in the control animals Importantly, clinical cancer progression driven by CSCs may
starting from week 3, while WA-treated animals exhibited contribute to the failure of both conventional and targeted
[4]
suppressed and restricted bioluminescent signal at the therapies. CSC is targeted by a novel fluorescent dye,
[22]
primary lesion site [left panel, Figure 6a]. Tumor burden was CDy1, which has specific affinity for pluripotent stem cells.
quantitatively measured as fold changes in bioluminescent Suspended F cells spontaneously formed tumor aggregates or
intensity and plotted against time [right panel, Figure 6a]. spheres under normal culture conditions and gave rise to A
In another model, when F cells were intravenously injected, cells with a greater differentiated phenotype, which have been
they appeared to localize to the abdominal region of the shown to be more sensitive to the conventional therapeutic
animals, and WA treatment appeared to prevent the spreading modalities, such as chemotherapy and/or radiotherapy. The
of the F cells [Figure 6b]. Subcutaneous tumor biopsies were dynamic phenotypic transition between F and A cells closely
obtained for immunohistochemical analysis. Immunostaining resembles CSC physiology, thereby representing an ideal in
Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ Issue 1 ¦ January 15, 2016 ¦ 37