Page 16 - Read Online
P. 16
Cheng et al. J Cancer Metastasis Treat 2021;7:17 https://dx.doi.org/10.20517/2394-4722.2021.27 Page 11 of 18
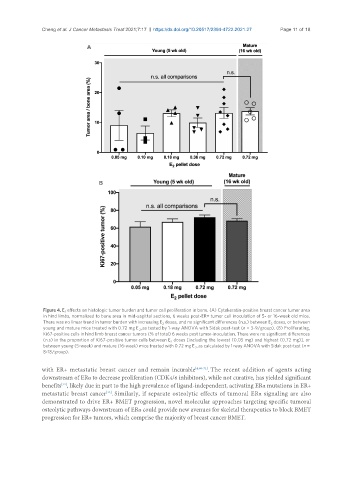
Figure 4. E effects on histologic tumor burden and tumor cell proliferation in bone. (A) Cytokeratin-positive breast cancer tumor area
2
in hind limbs, normalized to bone area in mid-sagittal sections, 6 weeks post-ER+ tumor cell inoculation of 5- or 16-week old mice.
There was no linear trend in tumor burden with increasing E doses, and no significant differences (n.s.) between E doses, or between
2 2
young and mature mice treated with 0.72 mg E , as tested by 1-way ANOVA with Sidak post-test (n = 3-9/group). (B) Proliferating,
2
Ki67-positive cells in hind limb breast cancer tumors (% of total) 6 weeks post tumor-inoculation. There were no significant differences
(n.s) in the proportion of Ki67-positive tumor cells between E doses [including the lowest (0.05 mg) and highest (0.72 mg)], or
2
between young (5-week) and mature (16-week) mice treated with 0.72 mg E , as calculated by 1-way ANOVA with Sidak post-test (n =
2
8-18/group).
with ER+ metastatic breast cancer and remain incurable [4,66-71] . The recent addition of agents acting
downstream of ERα to decrease proliferation (CDK4/6 inhibitors), while not curative, has yielded significant
benefits , likely due in part to the high prevalence of ligand-independent, activating ERα mutations in ER+
[72]
metastatic breast cancer . Similarly, if separate osteolytic effects of tumoral ERα signaling are also
[12]
demonstrated to drive ER+ BMET progression, novel molecular approaches targeting specific tumoral
osteolytic pathways downstream of ERα could provide new avenues for skeletal therapeutics to block BMET
progression for ER+ tumors, which comprise the majority of breast cancer BMET.