Page 17 - Read Online
P. 17
Page 12 of 18 Cheng et al. J Cancer Metastasis Treat 2021;7:17 https://dx.doi.org/10.20517/2394-4722.2021.27
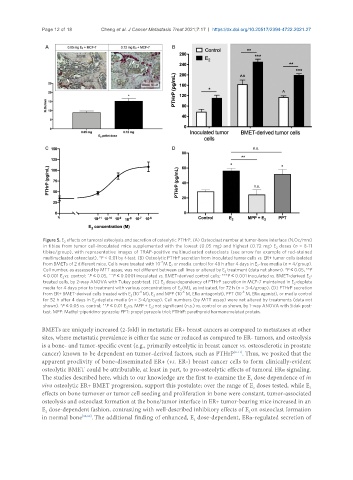
Figure 5. E effects on tumoral osteolysis and secretion of osteolytic PTHrP. (A) Osteoclast number at tumor-bone interface (N.Oc/mm)
2
in tibiae from tumor cell-inoculated mice supplemented with the lowest (0.05 mg) and highest (0.72 mg) E doses (n = 8-11
2
tibiae/group), with representative images of TRAP-positive multinucleated osteoclasts (see arrow for example of red-stained
multinucleated osteoclast). *P < 0.01 by t-test. (B) Osteolytic PTHrP secretion from inoculated tumor cells vs. ER+ tumor cells isolated
-7
from BMETs of 2 different mice. Cells were treated with 10 M E or media control for 48 h after 4 days in E -free media (n = 4/group).
2
2
Cell number, as assessed by MTT assay, was not different between cell lines or altered by E treatment (data not shown). *P ≤ 0.05, **P
2
≤ 0.001 E vs. control; ^P ≤ 0.05, ^^P ≤ 0.0001 inoculated vs. BMET-derived control cells; ***P ≤ 0.001 inoculated vs. BMET-derived E -
2
2
treated cells, by 2-way ANOVA with Tukey post-test. (C) E dose-dependency of PTHrP secretion in MCF-7 maintained in E -deplete
2
2
media for 4 days prior to treatment with various concentrations of E (M), as indicated, for 72 h (n = 3-4/group). (D) PTHrP secretion
2
-8
-8
-6
from ER+ BMET-derived cells treated with E (10 M), E and MPP (10 M, ERα antagonist), PPT (10 M, ERα agonist), or media control
2
2
for 52 h after 4 days in E -deplete media (n = 3-4/group). Cell numbers (by MTT assay) were not altered by treatments (data not
2
shown). *P ≤ 0.05 vs. control; **P ≤ 0.01 E vs. MPP + E ; not significant (n.s.) vs. control or as shown, by 1-way ANOVA with Sidak post-
2
2
test. MPP: Methyl-piperidino-pyrazole; PPT: propyl pyrazole triol; PTHrP: parathyroid hormone-related protein.
BMETs are uniquely increased (2-fold) in metastatic ER+ breast cancers as compared to metastases at other
sites, where metastatic prevalence is either the same or reduced as compared to ER- tumors, and osteolysis
is a bone- and tumor-specific event (e.g., primarily osteolytic in breast cancer vs. osteosclerotic in prostate
cancer) known to be dependent on tumor-derived factors, such as PTHrP [8-11] . Thus, we posited that the
apparent proclivity of bone-disseminated ER+ (vs. ER-) breast cancer cells to form clinically-evident
osteolytic BMET could be attributable, at least in part, to pro-osteolytic effects of tumoral ERα signaling.
The studies described here, which to our knowledge are the first to examine the E dose dependence of in
2
vivo osteolytic ER+ BMET progression, support this postulate; over the range of E doses tested, while E
2
2
effects on bone turnover or tumor cell seeding and proliferation in bone were constant, tumor-associated
osteolysis and osteoclast formation at the bone/tumor interface in ER+ tumor-bearing mice increased in an
E dose-dependent fashion, contrasting with well-described inhibitory effects of E on osteoclast formation
2
2
in normal bone [32,60] . The additional finding of enhanced, E dose-dependent, ERα-regulated secretion of
2