Page 149 - Read Online
P. 149
Page 10 of 13 Zhang et al. Energy Mater 2023;3:300008 https://dx.doi.org/10.20517/energymater.2022.71
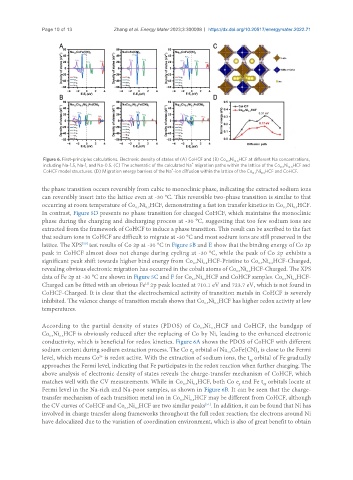
Figure 6. First-principles calculations. Electronic density of states of (A) CoHCF and (B) Co Ni HCF at different Na concentrations,
0.3
0.7
+
including Na-1.5, Na-1, and Na-0.5. (C) The schematic of the calculated Na migration paths within the lattice of the Co Ni HCF and
0.7
0.3
+
CoHCF model structures. (D) Migration energy barriers of the Na -ion diffusion within the lattice of the Co Ni HCF and CoHCF.
0.7
0.3
the phase transition occurs reversibly from cubic to monoclinic phase, indicating the extracted sodium ions
can reversibly insert into the lattice even at -30 °C. This reversible two-phase transition is similar to that
occurring at room temperature of Co Ni HCF, demonstrating a fast ion transfer kinetics in Co Ni HCF.
0.3
0.3
0.7
0.7
In contrast, Figure 5D presents no phase transition for charged CoHCF, which maintains the monoclinic
phase during the charging and discharging process at -30 °C, suggesting that too few sodium ions are
extracted from the framework of CoHCF to induce a phase transition. This result can be ascribed to the fact
that sodium ions in CoHCF are difficult to migrate at -30 °C and most sodium ions are still preserved in the
[50]
lattice. The XPS test results of Co 2p at -30 °C in Figure 5B and E show that the binding energy of Co 2p
peak in CoHCF almost does not change during cycling at -30 °C, while the peak of Co 2p exhibits a
significant peak shift towards higher bind energy from Co Ni HCF-Pristine to Co Ni HCF-Charged,
0.7
0.3
0.7
0.3
revealing obvious electronic migration has occurred in the cobalt atoms of Co Ni HCF-Charged. The XPS
0.3
0.7
data of Fe 2p at -30 °C are shown in Figure 5C and F for Co Ni HCF and CoHCF samples. Co Ni HCF-
0.3
0.7
0.7
0.3
Charged can be fitted with an obvious Fe 2p peak located at 710.1 eV and 723.7 eV, which is not found in
III
CoHCF-Charged. It is clear that the electrochemical activity of transition metals in CoHCF is severely
inhibited. The valence change of transition metals shows that Co Ni HCF has higher redox activity at low
0.3
0.7
temperatures.
According to the partial density of states (PDOS) of Co Ni HCF and CoHCF, the bandgap of
0.3
0.7
Co Ni HCF is obviously reduced after the replacing of Co by Ni, leading to the enhanced electronic
0.3
0.7
conductivity, which is beneficial for redox kinetics. Figure 6A shows the PDOS of CoHCF with different
sodium content during sodium extraction process. The Co e orbital of Na CoFe(CN) is close to the Fermi
g
1.5
6
level, which means Co is redox active. With the extraction of sodium ions, the t orbital of Fe gradually
2+
2g
approaches the Fermi level, indicating that Fe participates in the redox reaction when further charging. The
above analysis of electronic density of states reveals the charge-transfer mechanism of CoHCF, which
matches well with the CV measurements. While in Co Ni HCF, both Co e and Fe t orbitals locate at
2g
g
0.7
0.3
Fermi level in the Na-rich and Na-poor samples, as shown in Figure 6B. It can be seen that the charge-
transfer mechanism of each transition metal ion in Co Ni HCF may be different from CoHCF, although
0.7
0.3
[51]
the CV curves of CoHCF and Co Ni HCF are two similar peaks . In addition, it can be found that Ni has
0.7
0.3
involved in charge transfer along frameworks throughout the full redox reaction; the electrons around Ni
have delocalized due to the variation of coordination environment, which is also of great benefit to obtain