Page 146 - Read Online
P. 146
Zhang et al. Energy Mater 2023;3:300008 https://dx.doi.org/10.20517/energymater.2022.71 Page 7 of 13
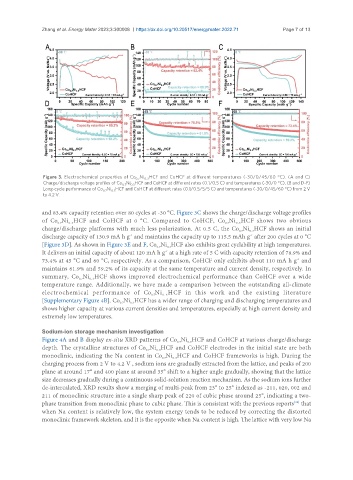
Figure 3. Electrochemical properties of Co Ni HCF and CoHCF at different temperatures (-30/0/45/60 °C). (A and C)
0.7 0.3
Charge/discharge voltage profiles of Co Ni HCF and CoHCF at different rates (0.1/0.5 C) and temperatures (-30/0 °C). (B and D-F)
0.7 0.3
Long-cycle performance of Co Ni HCF and CoHCF at different rates (0.1/0.5/5/5 C) and temperatures (-30/0/45/60 °C) from 2 V
0.7 0.3
to 4.2 V.
and 83.4% capacity retention over 80 cycles at -30 °C. Figure 3C shows the charge/discharge voltage profiles
of Co Ni HCF and CoHCF at 0 °C. Compared to CoHCF, Co Ni HCF shows two obvious
0.7
0.3
0.7
0.3
charge/discharge platforms with much less polarization. At 0.5 C, the Co Ni HCF shows an initial
0.7
0.3
-1
discharge capacity of 130.9 mA h g and maintains the capacity up to 115.5 mAh g after 200 cycles at 0 °C
-1
[Figure 3D]. As shown in Figure 3E and F, Co Ni HCF also exhibits great cyclability at high temperatures.
0.7
0.3
It delivers an initial capacity of about 120 mA h g at a high rate of 5 C with capacity retention of 78.9% and
-1
-1
73.4% at 45 °C and 60 °C, respectively. As a comparison, CoHCF only exhibits about 110 mA h g and
maintains 61.9% and 59.2% of its capacity at the same temperature and current density, respectively. In
summary, Co Ni HCF shows improved electrochemical performance than CoHCF over a wide
0.7
0.3
temperature range. Additionally, we have made a comparison between the outstanding all-climate
electrochemical performance of Co Ni HCF in this work and the existing literature
0.7
0.3
[Supplementary Figure 4B]. Co Ni HCF has a wider range of charging and discharging temperatures and
0.3
0.7
shows higher capacity at various current densities and temperatures, especially at high current density and
extremely low temperatures.
Sodium-ion storage mechanism investigation
Figure 4A and B display ex-situ XRD patterns of Co Ni HCF and CoHCF at various charge/discharge
0.7
0.3
depth. The crystalline structures of Co Ni HCF and CoHCF electrodes in the initial state are both
0.3
0.7
monoclinic, indicating the Na content in Co Ni HCF and CoHCF frameworks is high. During the
0.3
0.7
charging process from 2 V to 4.2 V , sodium ions are gradually extracted from the lattice, and peaks of 200
plane at around 17° and 400 plane at around 35° shift to a higher angle gradually, showing that the lattice
size decreases gradually during a continuous solid-solution reaction mechanism. As the sodium ions further
de-intercalated, XRD results show a merging of multi-peak from 23° to 25° indexed as -211, 020, 002 and
211 of monoclinic structure into a single sharp peak of 220 of cubic phase around 25°, indicating a two-
[39]
phase transition from monoclinic phase to cubic phase. This is consistent with the previous reports that
when Na content is relatively low, the system energy tends to be reduced by correcting the distorted
monoclinic framework skeleton, and it is the opposite when Na content is high. The lattice with very low Na