Page 145 - Read Online
P. 145
Page 6 of 13 Zhang et al. Energy Mater 2023;3:300008 https://dx.doi.org/10.20517/energymater.2022.71
-1
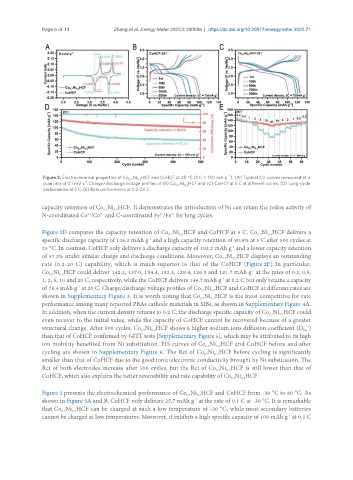
Figure 2. Electrochemical properties of Co Ni HCF and CoHCF at 25 °C (1 C = 150 mA g ). (A) Typical CV curves measured at a
0.7 0.3
-1
scan rate of 0.1 mV s . Charge/discharge voltage profiles of (B) Co Ni HCF and (C) CoHCF at 5 C at different cycles. (D) Long-cycle
0.7 0.3
performance at 5 C. (E) Rate performance at 0.2-20 C.
capacity retention of Co Ni HCF. It demonstrates the introduction of Ni can retain the redox activity of
0.3
0.7
3+
2+
2+
N-coordinated Co /Co and C-coordinated Fe /Fe for long cycles.
3+
Figure 2D compares the capacity retention of Co Ni HCF and CoHCF at 5 C. Co Ni HCF delivers a
0.3
0.7
0.7
0.3
-1
specific discharge capacity of 126.2 mAh g and a high capacity retention of 80.9% at 5 C after 500 cycles at
25 °C. In contrast, CoHCF only delivers a discharge capacity of 102.2 mAh g and a lower capacity retention
-1
of 57.2% under similar charge and discharge conditions. Moreover, Co Ni HCF displays an outstanding
0.3
0.7
rate (0.2-20 C) capability, which is much superior to that of the CoHCF [Figure 2E]. In particular,
Co Ni HCF could deliver 142.2, 137.0, 134.4, 132.3, 129.4, 126.3 and 121.7 mAh g at the rates of 0.2, 0.5,
-1
0.7
0.3
-1
1, 2, 5, 10 and 20 C, respectively, while the CoHCF delivers 149.7 mAh g at 0.2 C but only retains a capacity
of 78.4 mAh g at 20 C. Charge/discharge voltage profiles of Co Ni HCF and CoHCF at different rates are
-1
0.3
0.7
shown in Supplementary Figure 3. It is worth noting that Co Ni HCF is the most competitive for rate
0.7
0.3
performance among many reported PBAs cathode materials in SIBs, as shown in Supplementary Figure 4A.
In addition, when the current density returns to 0.2 C, the discharge specific capacity of Co Ni HCF could
0.3
0.7
even recover to the initial value, while the capacity of CoHCF cannot be recovered because of a greater
structural change. After 500 cycles, Co Ni HCF shows a higher sodium ions diffusion coefficient (D )
+
0.3
0.7
Na
than that of CoHCF confirmed by GITT tests [Supplementary Figure 5], which may be attributed to its high
ion mobility benefited from Ni substitution. EIS curves of Co Ni HCF and CoHCF before and after
0.7
0.3
cycling are shown in Supplementary Figure 6. The Rct of Co Ni HCF before cycling is significantly
0.3
0.7
smaller than that of CoHCF due to the good ionic/electronic conductivity brought by Ni substitution. The
Rct of both electrodes increase after 500 cycles, but the Rct of Co Ni HCF is still lower than that of
0.3
0.7
CoHCF, which also explains the better reversibility and rate capability of Co Ni HCF.
0.3
0.7
Figure 3 presents the electrochemical performance of Co Ni HCF and CoHCF from -30 °C to 60 °C. As
0.3
0.7
shown in Figure 3A and B, CoHCF only delivers 25.7 mAh g at the rate of 0.1 C at -30 °C. It is remarkable
-1
that Co Ni HCF can be charged at such a low temperature of -30 °C, while most secondary batteries
0.7
0.3
cannot be charged at low temperatures. Moreover, it exhibits a high specific capacity of 109 mAh g at 0.1 C
-1