Page 97 - Read Online
P. 97
Page 24 of 33 Mao et al. Chem Synth 2023;3:26 https://dx.doi.org/10.20517/cs.2022.41
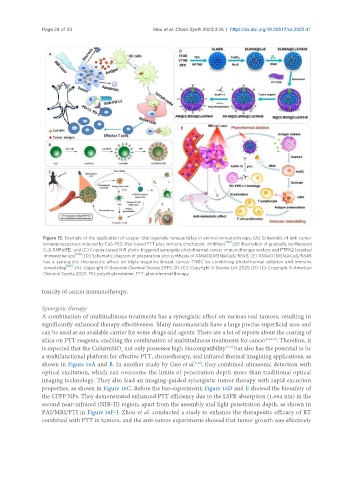
Figure 15. Example of the application of copper chalcogenide nanoparticles in animal immunotherapy. (A) Schematic of anti-tumor
immune responses induced by CuS-PEG-Mal-based PTT plus immune checkpoint inhibitors [153] ; (B) Illustration of gradually synthesized
CuS-RNP@PEI; and (C) Copper-based NIR photo-triggered synergetic photothermal cancer immunotherapy vectors and PTPN2 targeted
immunotherapy [188] ; (D) Schematic diagram of preparation and synthesis of AM@DLMSN@CuS/R848; (E) AM@DLMSN@CuS/R848
has a synergistic therapeutic effect on triple-negative breast cancer TNBC by combining photothermal ablation and immune
remodeling [149] . (A): Copyright © American Chemical Society 2019; (B)-(C): Copyright © Elsevier Ltd. 2021; (D)-(E): Copyright © American
Chemical Society 2020. PEI: polyethyleneimine; PTT: photothermal therapy.
toxicity of cancer immunotherapy.
Synergetic therapy
A combination of multitudinous treatments has a synergistic effect on various real tumors, resulting in
significantly enhanced therapy effectiveness. Many nanomaterials have a large precise superficial area and
can be used as an available carrier for some drugs and agents. There are a lot of reports about the coating of
silica on PTT reagents, enabling the combination of multitudinous treatments for cancer [74,150] . Therefore, it
is expected that the CuS@mSiO not only possesses high biocompatibility but also has the potential to be
[150]
2
a multifunctional platform for effective PTT, chemotherapy, and infrared thermal imagining applications, as
shown in Figure 16A and B. In another study by Guo et al. , they combined ultrasonic detection with
[138]
optical excitation, which can overcome the limits of penetration depth more than traditional optical
imaging technology. They also lead an imaging-guided synergistic tumor therapy with rapid excretion
properties, as shown in Figure 16C. Before the bio-experiment, Figure 16D and E showed the biosafety of
the CFPP NPs. They demonstrated enhanced PTT efficiency due to the LSPR absorption (1,064 nm) in the
second near-infrared (NIR-II) region, apart from the assembly and light penetration depth, as shown in
PAI/MRI/PTI in Figure 16F-J. Zhou et al. conducted a study to enhance the therapeutic efficacy of RT
combined with PTT in tumors, and the anti-tumor experiments showed that tumor growth was effectively