Page 26 - Read Online
P. 26
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 19 of 35
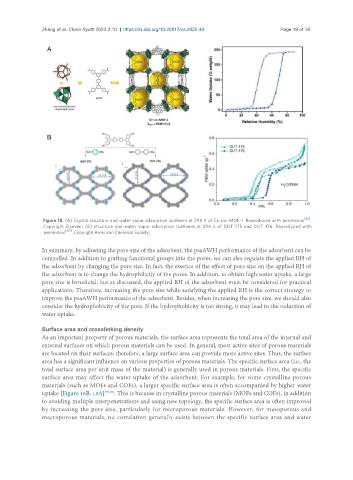
Figure 18. (A) Crystal structure and water vapor adsorption isotherm at 298 K of Cr-soc-MOF-1. Reproduced with permission [85] .
Copyright Elsevier; (B) structure and water vapor adsorption isotherm at 298 K of DUT-175 and DUT-176. Reproduced with
permission [107] . Copyright American Chemical Society.
In summary, by adjusting the pore size of the adsorbent, the psaAWH performance of the adsorbent can be
controlled. In addition to grafting functional groups into the pores, we can also regulate the applied RH of
the adsorbent by changing the pore size. In fact, the essence of the effect of pore size on the applied RH of
the adsorbent is to change the hydrophilicity of the pores. In addition, to obtain high water uptake, a large
pore size is beneficial, but as discussed, the applied RH of the adsorbent must be considered for practical
applications. Therefore, increasing the pore size while satisfying the applied RH is the correct strategy to
improve the psaAWH performance of the adsorbent. Besides, when increasing the pore size, we should also
consider the hydrophobicity of the pore. If the hydrophobicity is too strong, it may lead to the reduction of
water uptake.
Surface area and crosslinking density
As an important property of porous materials, the surface area represents the total area of the internal and
external surfaces on which porous materials can be used. In general, most active sites of porous materials
are located on their surfaces; therefore, a large surface area can provide more active sites. Thus, the surface
area has a significant influence on various properties of porous materials. The specific surface area (i.e., the
total surface area per unit mass of the material) is generally used in porous materials. First, the specific
surface area may affect the water uptake of the adsorbent. For example, for some crystalline porous
materials (such as MOFs and COFs), a larger specific surface area is often accompanied by higher water
uptake [Figure 16B, 18A] [79,85] . This is because in crystalline porous materials (MOFs and COFs), in addition
to avoiding multiple interpenetrations and using new topology, the specific surface area is often improved
by increasing the pore size, particularly for microporous materials. However, for mesoporous and
macroporous materials, no correlation generally exists between the specific surface area and water