Page 30 - Read Online
P. 30
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 23 of 35
Figure 21. Water vapor transport from air to brick through PEDOT microtubes. Reproduced with permission [154] . Copyright American
Chemical Society.
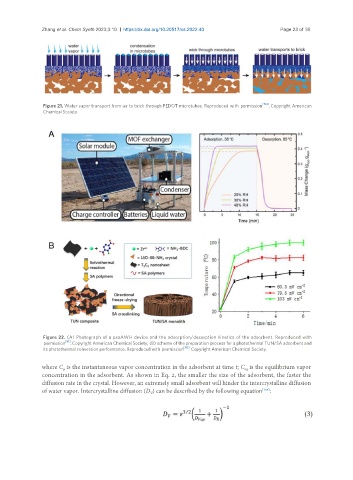
Figure 22. (A) Photograph of a psaAWH device and the adsorption/desorption kinetics of the adsorbent. Reproduced with
permission [91] . Copyright American Chemical Society; (B) scheme of the preparation process for a photothermal TUN/SA adsorbent and
its photothermal conversion performance. Reproduced with permission [86] . Copyright American Chemical Society.
where C is the instantaneous vapor concentration in the adsorbent at time t; C is the equilibrium vapor
eq
µ
concentration in the adsorbent. As shown in Eq. 2, the smaller the size of the adsorbent, the faster the
diffusion rate in the crystal. However, an extremely small adsorbent will hinder the intercrystalline diffusion
of water vapor. Intercrystalline diffusion (D ) can be described by the following equation :
[164]
V