Page 25 - Read Online
P. 25
Page 18 of 35 Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40
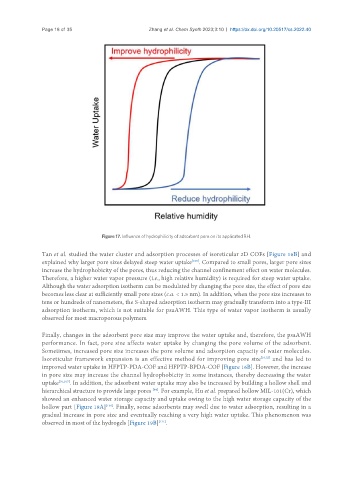
Figure 17. Influence of hydrophilicity of adsorbent pore on its applicated RH.
Tan et al. studied the water cluster and adsorption processes of isoreticular 2D COFs [Figure 16B] and
explained why larger pore sizes delayed steep water uptake . Compared to small pores, larger pore sizes
[100]
increase the hydrophobicity of the pores, thus reducing the channel confinement effect on water molecules.
Therefore, a higher water vapor pressure (i.e., high relative humidity) is required for steep water uptake.
Although the water adsorption isotherm can be modulated by changing the pore size, the effect of pore size
becomes less clear at sufficiently small pore sizes (c.a. < 1.5 nm). In addition, when the pore size increases to
tens or hundreds of nanometers, the S-shaped adsorption isotherm may gradually transform into a type-III
adsorption isotherm, which is not suitable for psaAWH. This type of water vapor isotherm is usually
observed for most macroporous polymers.
Finally, changes in the adsorbent pore size may improve the water uptake and, therefore, the psaAWH
performance. In fact, pore size affects water uptake by changing the pore volume of the adsorbent.
Sometimes, increased pore size increases the pore volume and adsorption capacity of water molecules.
Isoreticular framework expansion is an effective method for improving pore size [19,52] and has led to
improved water uptake in HFPTP-PDA-COF and HFPTP-BPDA-COF [Figure 16B]. However, the increase
in pore size may increase the channel hydrophobicity in some instances, thereby decreasing the water
uptake [76,107] . In addition, the adsorbent water uptake may also be increased by building a hollow shell and
hierarchical structure to provide large pores . For example, Hu et al. prepared hollow MIL-101(Cr), which
[84]
showed an enhanced water storage capacity and uptake owing to the high water storage capacity of the
hollow part [Figure 19A] . Finally, some adsorbents may swell due to water adsorption, resulting in a
[156]
gradual increase in pore size and eventually reaching a very high water uptake. This phenomenon was
observed in most of the hydrogels [Figure 19B] .
[121]