Page 27 - Read Online
P. 27
Page 20 of 35 Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40
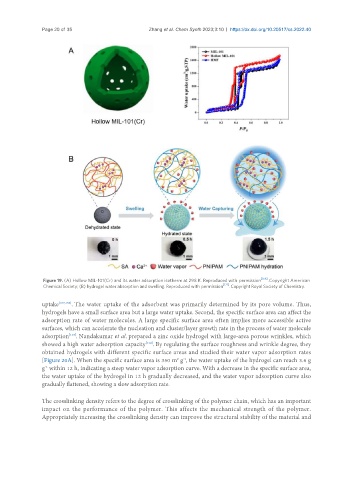
Figure 19. (A) Hollow MIL-101(Cr) and its water adsorption isotherm at 298 K. Reproduced with permission [156] . Copyright American
Chemical Society; (B) hydrogel water absorption and swelling. Reproduced with permission [121] . Copyright Royal Society of Chemistry.
uptake [107,156] . The water uptake of the adsorbent was primarily determined by its pore volume. Thus,
hydrogels have a small surface area but a large water uptake. Second, the specific surface area can affect the
adsorption rate of water molecules. A large specific surface area often implies more accessible active
surfaces, which can accelerate the nucleation and cluster/layer growth rate in the process of water molecule
adsorption . Nandakumar et al. prepared a zinc oxide hydrogel with large-area porous wrinkles, which
[113]
showed a high water adsorption capacity . By regulating the surface roughness and wrinkle degree, they
[114]
obtained hydrogels with different specific surface areas and studied their water vapor adsorption rates
[Figure 20A]. When the specific surface area is 350 m g , the water uptake of the hydrogel can reach 3.6 g
-1
2
g within 12 h, indicating a steep water vapor adsorption curve. With a decrease in the specific surface area,
-1
the water uptake of the hydrogel in 12 h gradually decreased, and the water vapor adsorption curve also
gradually flattened, showing a slow adsorption rate.
The crosslinking density refers to the degree of crosslinking of the polymer chain, which has an important
impact on the performance of the polymer. This affects the mechanical strength of the polymer.
Appropriately increasing the crosslinking density can improve the structural stability of the material and