Page 28 - Read Online
P. 28
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 21 of 35
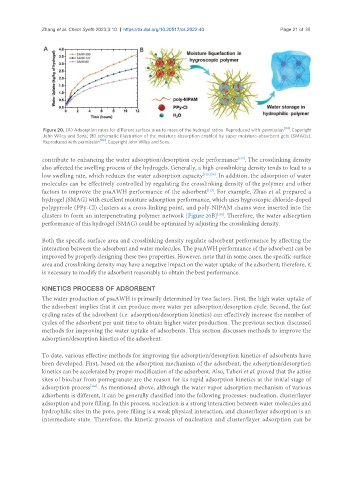
Figure 20. (A) Adsorption rates for different surface area to mass of the hydrogel ratios. Reproduced with permission [114] . Copyright
John Wiley and Sons; (B) schematic illustration of the moisture absorption enabled by super moisture-absorbent gels (SMAGs).
Reproduced with permission [116] . Copyright John Wiley and Sons.
contribute to enhancing the water adsorption/desorption cycle performance . The crosslinking density
[157]
also affected the swelling process of the hydrogels. Generally, a high crosslinking density tends to lead to a
low swelling rate, which reduces the water adsorption capacity [158,159] . In addition, the adsorption of water
molecules can be effectively controlled by regulating the crosslinking density of the polymer and other
factors to improve the psaAWH performance of the adsorbent . For example, Zhao et al. prepared a
[112]
hydrogel (SMAG) with excellent moisture adsorption performance, which uses hygroscopic chloride-doped
polypyrrole (PPy-Cl) clusters as a cross-linking point, and poly-NIPAM chains were inserted into the
clusters to form an interpenetrating polymer network [Figure 20B] . Therefore, the water adsorption
[116]
performance of this hydrogel (SMAG) could be optimized by adjusting the crosslinking density.
Both the specific surface area and crosslinking density regulate adsorbent performance by affecting the
interaction between the adsorbent and water molecules. The psaAWH performance of the adsorbent can be
improved by properly designing these two properties. However, note that in some cases, the specific surface
area and crosslinking density may have a negative impact on the water uptake of the adsorbent; therefore, it
is necessary to modify the adsorbent reasonably to obtain the best performance.
KINETICS PROCESS OF ADSORBENT
The water production of psaAWH is primarily determined by two factors. First, the high water uptake of
the adsorbent implies that it can produce more water per adsorption/desorption cycle. Second, the fast
cycling rates of the adsorbent (i.e. adsorption/desorption kinetics) can effectively increase the number of
cycles of the adsorbent per unit time to obtain higher water production. The previous section discussed
methods for improving the water uptake of adsorbents. This section discusses methods to improve the
adsorption/desorption kinetics of the adsorbent.
To date, various effective methods for improving the adsorption/desorption kinetics of adsorbents have
been developed. First, based on the adsorption mechanism of the adsorbent, the adsorption/desorption
kinetics can be accelerated by proper modification of the adsorbent. Also, Taheri et al. proved that the active
sites of biochar from pomegranate are the reason for its rapid adsorption kinetics at the initial stage of
adsorption process . As mentioned above, although the water vapor adsorption mechanism of various
[160]
adsorbents is different, it can be generally classified into the following processes: nucleation, cluster/layer
adsorption and pore filling. In this process, nucleation is a strong interaction between water molecules and
hydrophilic sites in the pore, pore filling is a weak physical interaction, and cluster/layer adsorption is an
intermediate state. Therefore, the kinetic process of nucleation and cluster/layer adsorption can be